Chem Unit7
Download as ppt, pdf0 likes361 views
The document discusses various topics relating to states of matter and gas laws. It defines the three common states of matter and phase transitions. It also explains the different types of intermolecular forces and how they relate to boiling points. Several gas laws are defined, including Boyle's, Charles', and Gay-Lussac's Laws. The ideal gas law and concepts like molar mass, density, diffusion, and partial pressures are also covered.
1 of 55
Downloaded 27 times
























Ad
Recommended
Gas Law
Gas LawAdmin Jan
╠²
The document discusses various gas laws and their applications, including Boyle's Law, Charles's Law, and Dalton's Law of partial pressures. It explains the physical characteristics of gases, calculations involving gas density, moles, and the ideal gas law (PV=nRT). The document also outlines the kinetic molecular theory of gases and deviations from ideal behavior.23 gases
23 gasesmrtangextrahelp
╠²
Gases are composed of molecules that are far apart from each other and occupy the entire volume of their container. Gas molecules move randomly in straight lines and collide elastically with each other and the container walls. The kinetic molecular theory describes gases as having negligible intermolecular forces and volume. Pressure, volume, temperature of a gas are related by the combined gas law and ideal gas law. Gas calculations involve using stoichiometry, the ideal gas law, and gas laws like Boyle's, Charles', and Gay-Lussac's to determine volume, pressure, amount, or temperature changes.Ideal gas law practice mccpot
Ideal gas law practice mccpotsohbel
╠²
1) The document discusses the ideal gas law (PV=nRT) and its applications in calculating things like moles of gas, volume at different conditions, density, and molar mass.
2) Key variables in the ideal gas law are defined such as pressure (P), volume (V), moles of gas (n), temperature (T), and the gas constant (R).
3) Standard temperature and pressure conditions are defined as 0┬░C and 1 atmosphere, where the molar volume of a gas is 22.4 L.Chemistry 107 Exam Prep 2
Chemistry 107 Exam Prep 2Kirsten Smirl
╠²
This document contains an agenda and notes for a chemistry class covering limiting reactants, acids and bases, and gas laws. The agenda includes:
- Limiting reactants
- Acids and bases
- Gas laws
- Questions
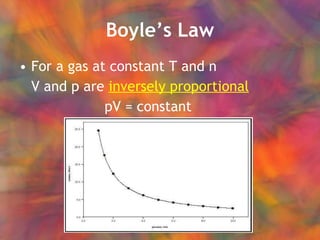
The notes provide examples and practice problems for determining limiting reactants, identifying strong and weak acids/bases, calculating neutralization reactions between acids and bases, and applying gas law equations like the ideal gas law, Boyle's law, Charles' law, and Avogadro's principle. Practice problems are included for determining gas properties based on temperature, pressure, volume, moles, and mass.Chem 101 week 12 ch 5
Chem 101 week 12 ch 5tdean1
╠²
Gases have unique characteristics compared to liquids and solids. They expand to fill their container and are highly compressible with low densities. To describe a gas, its volume, amount, temperature, and pressure must be specified. The behavior of gases is explained by kinetic molecular theory, which describes gases as particles in constant random motion. Real gases deviate from ideal behavior at high pressures and low temperatures due to intermolecular forces. The van der Waals equation accounts for these non-ideal effects.Ch5 z5e gases
Ch5 z5e gasesblachman
╠²
The document summarizes key gas laws including Boyle's law, Charles' law, Avogadro's law, Dalton's law of partial pressures, and the ideal gas law. It provides examples of using these laws to calculate volume, pressure, temperature, moles, and mass in gas reactions and mixtures. Key relationships covered are that pressure and volume are inversely related at constant temperature (Boyle's law), volume and temperature are directly related at constant pressure (Charles' law), and volume and moles are directly related at constant temperature and pressure (Avogadro's law).12 Gas Laws
12 Gas Lawsjanetra
╠²
The document summarizes 12 gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas law. It provides examples of calculations using these laws to determine moles of gas, volumes at different temperatures and pressures, and identities of gases based on density. Key formulas covered are PV=nRT, relationships between volume, pressure and temperature, and stoichiometric calculations using gas volumes.Gas Laws
Gas LawsMa Ann Charmaine Bernardino
╠²
The document describes several key concepts relating to gases:
1) It outlines the postulates of kinetic molecular theory and how they describe the behavior of ideal gases.
2) It then discusses how real gases differ from ideal gases and how their behavior is affected by factors like pressure and temperature.
3) Several gas laws are introduced that describe the relationships between pressure, volume, temperature, and number of moles for gases including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, Dalton's law of partial pressures, and the ideal gas law.Lec5 a
Lec5 aMohammed AlMujaini
╠²
Here are the key steps to solve this problem using Charles' Law:
1) Convert temperatures to Kelvin:
T1 = 297.0 K
T2 = 216.5 K
2) Use the Charles' Law equation:
V1/T1 = V2/T2
3) Solve for V2:
V2 = V1 * T2/T1
= 20.0 L * 216.5 K/297.0 K
= 14.6 L
So the volume outside at -70┬░F would be 14.6 L.Gas law
Gas lawPhß║Īm Ho├Āng
╠²
The document is the notes from a student, Le Kim Hoang Pham, for an oral exam on gas laws. It covers the key gas properties of pressure, volume, temperature, and amount. It summarizes the empirical gas laws discovered by Boyle, Charles, Gay-Lussac, Avogadro and describes how they relate the gas properties. It also presents the ideal gas law which combines these relationships into a single equation. Finally, it discusses gas mixtures and Dalton's law of partial pressures.New chm 151_unit_10_power_points
New chm 151_unit_10_power_pointscaneman1
╠²
This document provides an overview of gas laws and the behavior of gases. It begins by defining the three states of matter and distinguishing properties of gases. Gas pressure and its measurement are then discussed, including common pressure units. The document outlines the major gas laws - Boyle's Law relating pressure and volume at constant temperature, Charles' Law relating volume and temperature at constant pressure, and the Combined Gas Law combining these relationships. Examples are provided to demonstrate applications of the gas laws. The ideal gas law is defined as relating pressure, volume, temperature, and moles of gas. The behavior of gases at standard temperature and pressure is also covered.Chapter 5 gases reduced1
Chapter 5 gases reduced1mtsaeed03
╠²
This chapter discusses the key concepts and gas laws relating to gases:
1) Boyle's law describes the inverse relationship between pressure and volume at constant temperature.
2) Charles' law explains that gas volume increases with temperature at constant pressure.
3) Avogadro's law states that equal volumes of gases under the same conditions contain equal numbers of molecules.
4) The ideal gas law combines these relationships to quantitatively relate the pressure, volume, temperature, and amount of an ideal gas.Ch6 Thermochemistry (updated)
Ch6 Thermochemistry (updated)Sa'ib J. Khouri
╠²
Thermochemistry is the study of heat changes in chemical reactions. There are several types of energy including chemical, thermal, nuclear, and radiant energy. Heat is the transfer of thermal energy between objects at different temperatures. Thermochemistry examines heat absorbed or released by chemical reactions using concepts like exothermic, endothermic, enthalpy, and calorimetry. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred between systems and their surroundings.AP Physics - Chapter 14 Powerpoint
AP Physics - Chapter 14 PowerpointMrreynon
╠²
The document discusses the ideal gas law and kinetic theory of gases. It introduces concepts like the mole, Avogadro's number, molecular mass, and how these relate to the ideal gas law. The ideal gas law states that the absolute pressure of an ideal gas is directly proportional to temperature, number of moles, and inversely proportional to volume. Kinetic theory explains gas properties by considering gases as large numbers of constantly moving molecules, with the average molecular kinetic energy related to temperature.Thermodynamics objective
Thermodynamics objectivesuresh gdvm
╠²
1. The document contains a practice exam with 37 multiple choice questions covering concepts in thermodynamics and chemistry. The questions cover topics like ideal gases, enthalpy, entropy, spontaneity of reactions, and more.
2. For each question there are 4 possible answers labeled a-d. The correct answers are not provided.
3. The questions are intended to test understanding of fundamental thermodynamic concepts and calculations involving things like heat, work, internal energy, and state functions.Ch 14 Ideal Gas Law & Kinetic Theory
Ch 14 Ideal Gas Law & Kinetic TheoryScott Thomas
╠²
The document summarizes key concepts from Chapter 14 on the ideal gas law and kinetic theory. Section 1 discusses molecular mass, the mole, and Avogadro's number. Section 2 covers the ideal gas law and how pressure, volume, temperature, and moles are related. Section 3 introduces the kinetic theory model, which describes gases as large numbers of constantly moving particles and explains gas properties and behaviors in terms of particle collisions and kinetic energy.Chemical Thermodynamics
Chemical Thermodynamics suresh gdvm
╠²
Thermodynamics is the study of heat and work, and state functions. Energy exists in various forms including heat, light, electrical, and kinetic and potential. Heat is the transfer of energy between objects due to a temperature difference. Chemical reactions can be exothermic or endothermic depending on the direction of heat transfer. The total energy in a chemical system is conserved according to the law of conservation of energy. Changes in potential and kinetic energy account for temperature changes in chemical processes.Ap Chem: Unit 5: Gases
Ap Chem: Unit 5: Gasesbobcatchemistry
╠²
The document discusses key concepts about gases from the kinetic molecular theory and gas laws. It introduces gases in the atmosphere and how they were studied historically. It then covers gas pressure, units of pressure, Boyle's law, Charles' law, Avogadro's law, the ideal gas law, gas stoichiometry, Dalton's law of partial pressures, and the kinetic molecular theory of gases. Examples are provided to demonstrate calculations using these gas laws and concepts.Thermodynamics kinetics
Thermodynamics kineticsselvakumar948
╠²
1) Thermodynamics describes how changes in temperature, pressure, and composition affect equilibrium in rock systems. It allows prediction of mineral stability and interpretation of mineral compositions.
2) Thermodynamic models use concepts like internal energy, enthalpy, entropy, and Gibbs free energy to describe equilibrium and the direction of spontaneous processes.
3) Phase diagrams visualize thermodynamic stability by showing pressure-temperature-composition conditions where minerals are stable. However, kinetic effects can cause disequilibrium.AP Chemistry Chapter 10 Outline
AP Chemistry Chapter 10 OutlineJane Hamze
╠²
Gases are highly compressible and expand to fill their containers, with pressure inversely proportional to volume according to Boyle's Law. The properties and behavior of gases can be explained by the kinetic molecular theory, which models gases as large numbers of molecules in random motion. Real gases deviate from ideal gas behavior at high pressures and low temperatures due to intermolecular forces and molecular volumes.01 part1-ideal-gas
01 part1-ideal-gasgunabalan sellan
╠²
This document discusses ideal gases and their properties. It defines an ideal gas as a theoretical gas composed of point-like particles that obey gas laws perfectly. Some key properties of ideal gases are that they have negligible volume and mass and undergo perfectly elastic collisions. The document also covers gas laws such as Boyle's, Charles', and Gay-Lussac's laws. It provides the ideal gas equation of state and defines terms like molar mass, gas constant, pressure units and heat capacities. Several example problems applying the ideal gas law are also included.Ideal Gas Law
Ideal Gas LawJan Parker
╠²
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. The ideal gas law combines Boyle's, Charles', Gay-Lussac's and Avogadro's gas laws into one equation, PV=nRT, which relates the pressure (P), volume (V), number of moles (n), temperature (T) of an ideal gas. The ideal gas law accurately describes gas behavior at normal room temperature and pressure but real gases deviate from ideal behavior at high pressures due to intermolecular forces or at low temperatures where particle volume is significant.gaseous state
gaseous statesuresh gdvm
╠²
The document contains multiple choice questions about gas laws, kinetic molecular theory, and properties of gases.
1) Questions ask about calculating properties like density and pressure given temperature, volume, amount of gas, and other variables.
2) Other questions relate to concepts like effusion rates, van der Waals constants, and the relationship between temperature, pressure, volume, and number of gas molecules.
3) Graphs and diagrams are included that must be interpreted in the context of gas behavior and equations of state.The Ideal Gas Law
The Ideal Gas Lawguest1a9ef1b
╠²
The document discusses the ideal gas law PV=nRT and its application to real gases. It defines the four variables in the equation, describes how the ideal gas law relates these variables, and explains how to derive the gas constant R. While real gases deviate from ideal behavior, the ideal gas law can still adequately describe gases at low pressures and temperatures near room conditions. Two examples are given applying the law to calculate pressure and temperature for gas samples.Chem unit 12 presentation
Chem unit 12 presentationbobcatchemistry
╠²
Here is a one page paper relating chemistry and gases:
Chemistry and gases are intimately related. Many of the most important discoveries and applications in chemistry involve gases. Historically, scientists like Robert Boyle, Jacques Charles, and Joseph Gay-Lussac made seminal discoveries about gas behavior through careful experimentation. Their gas laws laid the foundation for understanding the properties and interactions of gases.
One area where gases play a huge role is in industry and energy. The Haber process converts nitrogen gas and hydrogen gas into ammonia, a key component of fertilizers that have enabled the growth of the global population. Natural gas, composed primarily of methane, heats homes and fuels power plants around the world. Greenhouse gases like carbon dioxideGas laws
Gas lawsWaylie Ni├▒a De Claro
╠²
The document discusses gas laws and properties of gases. It provides examples of problems involving Boyle's law, Charles' law, Gay-Lussac's law, combined gas law, and the ideal gas law. It also discusses Avogadro's law and the kinetic molecular theory of gases. Examples include calculating gas pressures, volumes, temperatures, and moles of gas under varying conditions.Gas
Gasfhugob
╠²
- Gases exhibit behaviors described by gas laws such as Boyle's law, Charles' law, and Gay-Lussac's law. The ideal gas law combines several gas laws into a single equation relating pressure, volume, temperature, and moles of gas.
- Real gases deviate from ideal gas behavior due to intermolecular forces and molecular size. The van der Waals equation accounts for these factors.
- Kinetic molecular theory explains gas properties in terms of the high-speed motion and infrequent collisions of molecules separated by large distances.Gases
GasesJaypee Baylosis
╠²
The document discusses the properties and behavior of gases, liquids, and solids from a microscopic perspective. It explains that gases have particles with empty space between them allowing the particles to move freely, while liquids have particles close together allowing them to flow but slide past one another, and solids have particles locked in a fixed structure. The document also covers gas laws, phase changes, types of solids based on bonding, and heating curves. It provides a concise overview of the states of matter and changes between states from a molecular level.Gases
GasesLumen Learning
╠²
This document provides an overview of key concepts related to gases, including:
- The properties of ideal gases and units of pressure like pascals and atmospheres.
- Gas laws including Boyle's, Charles', Gay-Lussac's, Avogadro's, and the ideal gas law.
- Conditions of STP and using gas laws to perform stoichiometric calculations.
- The kinetic molecular theory which explains gas properties in terms of particle motion.
- How real gases differ from ideal gases due to intermolecular forces.Chapter 5 notes
Chapter 5 notesWong Hsiung
╠²
Gases exist as individual molecules that are in constant random motion. The kinetic molecular theory describes gases as composed of molecules that are separated by large distances and move rapidly in random directions, frequently colliding with one another. The theory states that the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas. Higher temperatures cause molecules to move faster on average with more molecules possessing higher speeds.More Related Content
What's hot (18)
Lec5 a
Lec5 aMohammed AlMujaini
╠²
Here are the key steps to solve this problem using Charles' Law:
1) Convert temperatures to Kelvin:
T1 = 297.0 K
T2 = 216.5 K
2) Use the Charles' Law equation:
V1/T1 = V2/T2
3) Solve for V2:
V2 = V1 * T2/T1
= 20.0 L * 216.5 K/297.0 K
= 14.6 L
So the volume outside at -70┬░F would be 14.6 L.Gas law
Gas lawPhß║Īm Ho├Āng
╠²
The document is the notes from a student, Le Kim Hoang Pham, for an oral exam on gas laws. It covers the key gas properties of pressure, volume, temperature, and amount. It summarizes the empirical gas laws discovered by Boyle, Charles, Gay-Lussac, Avogadro and describes how they relate the gas properties. It also presents the ideal gas law which combines these relationships into a single equation. Finally, it discusses gas mixtures and Dalton's law of partial pressures.New chm 151_unit_10_power_points
New chm 151_unit_10_power_pointscaneman1
╠²
This document provides an overview of gas laws and the behavior of gases. It begins by defining the three states of matter and distinguishing properties of gases. Gas pressure and its measurement are then discussed, including common pressure units. The document outlines the major gas laws - Boyle's Law relating pressure and volume at constant temperature, Charles' Law relating volume and temperature at constant pressure, and the Combined Gas Law combining these relationships. Examples are provided to demonstrate applications of the gas laws. The ideal gas law is defined as relating pressure, volume, temperature, and moles of gas. The behavior of gases at standard temperature and pressure is also covered.Chapter 5 gases reduced1
Chapter 5 gases reduced1mtsaeed03
╠²
This chapter discusses the key concepts and gas laws relating to gases:
1) Boyle's law describes the inverse relationship between pressure and volume at constant temperature.
2) Charles' law explains that gas volume increases with temperature at constant pressure.
3) Avogadro's law states that equal volumes of gases under the same conditions contain equal numbers of molecules.
4) The ideal gas law combines these relationships to quantitatively relate the pressure, volume, temperature, and amount of an ideal gas.Ch6 Thermochemistry (updated)
Ch6 Thermochemistry (updated)Sa'ib J. Khouri
╠²
Thermochemistry is the study of heat changes in chemical reactions. There are several types of energy including chemical, thermal, nuclear, and radiant energy. Heat is the transfer of thermal energy between objects at different temperatures. Thermochemistry examines heat absorbed or released by chemical reactions using concepts like exothermic, endothermic, enthalpy, and calorimetry. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred between systems and their surroundings.AP Physics - Chapter 14 Powerpoint
AP Physics - Chapter 14 PowerpointMrreynon
╠²
The document discusses the ideal gas law and kinetic theory of gases. It introduces concepts like the mole, Avogadro's number, molecular mass, and how these relate to the ideal gas law. The ideal gas law states that the absolute pressure of an ideal gas is directly proportional to temperature, number of moles, and inversely proportional to volume. Kinetic theory explains gas properties by considering gases as large numbers of constantly moving molecules, with the average molecular kinetic energy related to temperature.Thermodynamics objective
Thermodynamics objectivesuresh gdvm
╠²
1. The document contains a practice exam with 37 multiple choice questions covering concepts in thermodynamics and chemistry. The questions cover topics like ideal gases, enthalpy, entropy, spontaneity of reactions, and more.
2. For each question there are 4 possible answers labeled a-d. The correct answers are not provided.
3. The questions are intended to test understanding of fundamental thermodynamic concepts and calculations involving things like heat, work, internal energy, and state functions.Ch 14 Ideal Gas Law & Kinetic Theory
Ch 14 Ideal Gas Law & Kinetic TheoryScott Thomas
╠²
The document summarizes key concepts from Chapter 14 on the ideal gas law and kinetic theory. Section 1 discusses molecular mass, the mole, and Avogadro's number. Section 2 covers the ideal gas law and how pressure, volume, temperature, and moles are related. Section 3 introduces the kinetic theory model, which describes gases as large numbers of constantly moving particles and explains gas properties and behaviors in terms of particle collisions and kinetic energy.Chemical Thermodynamics
Chemical Thermodynamics suresh gdvm
╠²
Thermodynamics is the study of heat and work, and state functions. Energy exists in various forms including heat, light, electrical, and kinetic and potential. Heat is the transfer of energy between objects due to a temperature difference. Chemical reactions can be exothermic or endothermic depending on the direction of heat transfer. The total energy in a chemical system is conserved according to the law of conservation of energy. Changes in potential and kinetic energy account for temperature changes in chemical processes.Ap Chem: Unit 5: Gases
Ap Chem: Unit 5: Gasesbobcatchemistry
╠²
The document discusses key concepts about gases from the kinetic molecular theory and gas laws. It introduces gases in the atmosphere and how they were studied historically. It then covers gas pressure, units of pressure, Boyle's law, Charles' law, Avogadro's law, the ideal gas law, gas stoichiometry, Dalton's law of partial pressures, and the kinetic molecular theory of gases. Examples are provided to demonstrate calculations using these gas laws and concepts.Thermodynamics kinetics
Thermodynamics kineticsselvakumar948
╠²
1) Thermodynamics describes how changes in temperature, pressure, and composition affect equilibrium in rock systems. It allows prediction of mineral stability and interpretation of mineral compositions.
2) Thermodynamic models use concepts like internal energy, enthalpy, entropy, and Gibbs free energy to describe equilibrium and the direction of spontaneous processes.
3) Phase diagrams visualize thermodynamic stability by showing pressure-temperature-composition conditions where minerals are stable. However, kinetic effects can cause disequilibrium.AP Chemistry Chapter 10 Outline
AP Chemistry Chapter 10 OutlineJane Hamze
╠²
Gases are highly compressible and expand to fill their containers, with pressure inversely proportional to volume according to Boyle's Law. The properties and behavior of gases can be explained by the kinetic molecular theory, which models gases as large numbers of molecules in random motion. Real gases deviate from ideal gas behavior at high pressures and low temperatures due to intermolecular forces and molecular volumes.01 part1-ideal-gas
01 part1-ideal-gasgunabalan sellan
╠²
This document discusses ideal gases and their properties. It defines an ideal gas as a theoretical gas composed of point-like particles that obey gas laws perfectly. Some key properties of ideal gases are that they have negligible volume and mass and undergo perfectly elastic collisions. The document also covers gas laws such as Boyle's, Charles', and Gay-Lussac's laws. It provides the ideal gas equation of state and defines terms like molar mass, gas constant, pressure units and heat capacities. Several example problems applying the ideal gas law are also included.Ideal Gas Law
Ideal Gas LawJan Parker
╠²
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. The ideal gas law combines Boyle's, Charles', Gay-Lussac's and Avogadro's gas laws into one equation, PV=nRT, which relates the pressure (P), volume (V), number of moles (n), temperature (T) of an ideal gas. The ideal gas law accurately describes gas behavior at normal room temperature and pressure but real gases deviate from ideal behavior at high pressures due to intermolecular forces or at low temperatures where particle volume is significant.gaseous state
gaseous statesuresh gdvm
╠²
The document contains multiple choice questions about gas laws, kinetic molecular theory, and properties of gases.
1) Questions ask about calculating properties like density and pressure given temperature, volume, amount of gas, and other variables.
2) Other questions relate to concepts like effusion rates, van der Waals constants, and the relationship between temperature, pressure, volume, and number of gas molecules.
3) Graphs and diagrams are included that must be interpreted in the context of gas behavior and equations of state.The Ideal Gas Law
The Ideal Gas Lawguest1a9ef1b
╠²
The document discusses the ideal gas law PV=nRT and its application to real gases. It defines the four variables in the equation, describes how the ideal gas law relates these variables, and explains how to derive the gas constant R. While real gases deviate from ideal behavior, the ideal gas law can still adequately describe gases at low pressures and temperatures near room conditions. Two examples are given applying the law to calculate pressure and temperature for gas samples.Chem unit 12 presentation
Chem unit 12 presentationbobcatchemistry
╠²
Here is a one page paper relating chemistry and gases:
Chemistry and gases are intimately related. Many of the most important discoveries and applications in chemistry involve gases. Historically, scientists like Robert Boyle, Jacques Charles, and Joseph Gay-Lussac made seminal discoveries about gas behavior through careful experimentation. Their gas laws laid the foundation for understanding the properties and interactions of gases.
One area where gases play a huge role is in industry and energy. The Haber process converts nitrogen gas and hydrogen gas into ammonia, a key component of fertilizers that have enabled the growth of the global population. Natural gas, composed primarily of methane, heats homes and fuels power plants around the world. Greenhouse gases like carbon dioxideGas laws
Gas lawsWaylie Ni├▒a De Claro
╠²
The document discusses gas laws and properties of gases. It provides examples of problems involving Boyle's law, Charles' law, Gay-Lussac's law, combined gas law, and the ideal gas law. It also discusses Avogadro's law and the kinetic molecular theory of gases. Examples include calculating gas pressures, volumes, temperatures, and moles of gas under varying conditions.Similar to Chem Unit7 (20)
Gas
Gasfhugob
╠²
- Gases exhibit behaviors described by gas laws such as Boyle's law, Charles' law, and Gay-Lussac's law. The ideal gas law combines several gas laws into a single equation relating pressure, volume, temperature, and moles of gas.
- Real gases deviate from ideal gas behavior due to intermolecular forces and molecular size. The van der Waals equation accounts for these factors.
- Kinetic molecular theory explains gas properties in terms of the high-speed motion and infrequent collisions of molecules separated by large distances.Gases
GasesJaypee Baylosis
╠²
The document discusses the properties and behavior of gases, liquids, and solids from a microscopic perspective. It explains that gases have particles with empty space between them allowing the particles to move freely, while liquids have particles close together allowing them to flow but slide past one another, and solids have particles locked in a fixed structure. The document also covers gas laws, phase changes, types of solids based on bonding, and heating curves. It provides a concise overview of the states of matter and changes between states from a molecular level.Gases
GasesLumen Learning
╠²
This document provides an overview of key concepts related to gases, including:
- The properties of ideal gases and units of pressure like pascals and atmospheres.
- Gas laws including Boyle's, Charles', Gay-Lussac's, Avogadro's, and the ideal gas law.
- Conditions of STP and using gas laws to perform stoichiometric calculations.
- The kinetic molecular theory which explains gas properties in terms of particle motion.
- How real gases differ from ideal gases due to intermolecular forces.Chapter 5 notes
Chapter 5 notesWong Hsiung
╠²
Gases exist as individual molecules that are in constant random motion. The kinetic molecular theory describes gases as composed of molecules that are separated by large distances and move rapidly in random directions, frequently colliding with one another. The theory states that the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas. Higher temperatures cause molecules to move faster on average with more molecules possessing higher speeds.Gas law and partial pressures
Gas law and partial pressuresFelipe De la Garza
╠²
The document summarizes key concepts of gas laws and gas behavior:
1. Gases assume the volume and shape of their container, are highly compressible, and mix completely. The ideal gas law relates the pressure, volume, amount, and temperature of a gas.
2. Boyle's, Charles', and Avogadro's laws describe how gas properties change with pressure, temperature, and amount at constant conditions. Dalton's law explains partial pressures in gas mixtures.
3. Real gases deviate from ideal behavior due to intermolecular forces, following the same trends but requiring correction factors at high pressures or low temperatures.General chemistry Chapter 3_may 9_2023.pptx
General chemistry Chapter 3_may 9_2023.pptxYilkal Dessie (ßŗŁßłŹßēāßłŹ ßŗ░ßł┤)
╠²
The document discusses the kinetic molecular theory of gases, defining comparisons between gases, vapors, and their characteristics. It explains gas laws, including Boyle's, Charles's, and Avogadro's laws, and their mathematical relationships while also covering the ideal gas equation. Additionally, it touches on intermolecular forces, properties of liquids and solids, and phase changes, concluding with solutions and solubility concepts.the gaseous state of matter
the gaseous state of matterUNIVERSITY OF JOHANNESBURG
╠²
1) When a hot air balloon is heated, some of the air escapes from the top, lowering the density inside and making the balloon buoyant.
2) The atmosphere protects the planet and provides chemicals necessary for life, including oxygen for metabolism, nitrogen to dilute oxygen, and carbon dioxide and water vapor that trap heat.
3) Gases have indefinite volume and shape, low densities, and high kinetic energies according to their temperature as described by the kinetic molecular theory.ch5.pptx
ch5.pptxaliabdolkhani4
╠²
The document discusses the properties and laws of gases, including their physical characteristics, pressure, volume, and temperature relationships as governed by Boyle's, Charles's, and Avogadro's laws. It also covers the ideal gas law, stoichiometry of gases, Dalton's law of partial pressures, and kinetic molecular theory. Additionally, it examines deviations from ideal gas behavior and practical applications in scenarios such as scuba diving and gas effusion.Gases.pptx
Gases.pptxFatmaMoustafa6
╠²
Gases are composed of molecules that are widely separated and in constant, rapid motion. This motion causes gases to exert pressure on their container walls and diffuse freely. The state of a gas is defined by its volume, pressure, temperature, and amount. Under normal conditions, gases approximate ideal gas behavior described by the ideal gas law (PV=nRT), though deviations occur at high pressures and low temperatures when molecular interactions are significant. Common gases in the atmosphere and human body include nitrogen, oxygen, carbon dioxide and their behaviors and roles are described.Ch5 Gases
Ch5 GasesSa'ib J. Khouri
╠²
1. Gases have no definite shape or volume but take the shape of their container. Gas particles are in constant random motion and collide with each other and the container walls.
2. The kinetic molecular theory provides an explanation for gas behavior at the molecular level. It states that gas particles are in constant random motion and exert pressure due to collisions with container walls.
3. The gas laws describe the macroscopic behavior of gases through relationships between pressure, volume, temperature, and amount of gas. The kinetic molecular theory qualitatively explains the gas laws based on gas particle motion and interactions.Gas Laws
Gas LawsRegis Komperda
╠²
The document discusses the kinetic molecular theory and properties of gases. It describes how gas particles are in constant random motion and how their speed is related to temperature. It also explains gas laws including Boyle's law, Charles' law, Gay-Lussac's law, Dalton's law of partial pressures, and diffusion and effusion of gas particles.States of matter
States of matterHoshi94
╠²
1. The document discusses the different states of matter and summarizes the key differences between gases, liquids, and solids.
2. It then covers various gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas equation.
3. The kinetic molecular theory is introduced to explain gas behavior at the molecular level in terms of molecule motion and interactions.GAS LAWS AND GENERAL GAS EQUATION (CHARLES, BOYLES AND AVOGADRO)
GAS LAWS AND GENERAL GAS EQUATION (CHARLES, BOYLES AND AVOGADRO)awanfenegideon98
╠²
The document provides an overview of gases, covering their physical characteristics, laws governing gas behavior (such as Boyle's, Charles', and Avogadro's laws), and the ideal gas equation. It discusses calculations related to gas volumes, pressures, and densities under various conditions, including standard temperature and pressure (STP). Additionally, it explores concepts like the kinetic molecular theory and Dalton's law of partial pressures.chap 2 gases in atmosphere.ppt bilan des flux gazeux
chap 2 gases in atmosphere.ppt bilan des flux gazeuxNouhailadark
╠²
The document discusses the properties and behaviors of gases in the atmosphere, including pressure measurement, the kinetic molecular theory, and various gas laws like Boyle's, Charles's, and Avogadro's laws. It also covers the ideal gas law, Dalton's law of partial pressures, and the collection of gases over water, providing equations and constants relevant to the calculations. Additionally, it addresses the differences between ideal and real gases and introduces the Van der Waals equation for describing real gas behavior.Ch5 Gases
Ch5 GasesSa'ib J. Khouri
╠²
1. Gases have certain physical properties according to the kinetic molecular theory including occupying the shape and volume of their container, being highly compressible, and mixing evenly.
2. The gas laws describe the relationships between pressure, volume, temperature, and amount of gas including Boyle's law, Charles' law, Avogadro's law, and the combined ideal gas law.
3. Real gases deviate from ideal behavior at high pressures as described by the van der Waals equation.Chapter 14 Gas Laws ppt 2017 good (1).ppt
Chapter 14 Gas Laws ppt 2017 good (1).pptmikeebio1
╠²
The document discusses kinetic molecular theory and the gas laws. It explains that kinetic molecular theory views gases as made of particles in constant, random motion that explains gas properties like pressure and temperature. The gas laws described are Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, Dalton's law of partial pressures, and the combined and ideal gas laws. Key concepts are that gas pressure, volume, and temperature are directly or inversely proportional based on these laws. Standard temperature and pressure are defined, and gas calculations can be done using the various gas laws and stoichiometry.Chapter_5_Gases.ppt
Chapter_5_Gases.pptgiovanimaguin7
╠²
The document summarizes key concepts about gases including:
1. Gases assume the volume and shape of their containers, are highly compressible, and will mix completely in the same container.
2. Gas pressure and volume are inversely related according to Boyle's Law. Temperature and volume are directly related according to Charles' Law.
3. The ideal gas law describes the relationship between pressure, volume, temperature, and moles of gas as PV=nRT.pptnotes 14 gas laws glembocki.pptx
pptnotes 14 gas laws glembocki.pptxJomarDeray1
╠²
Boyle's law states that at a constant temperature, the pressure and volume of a gas are inversely proportional. The product of pressure and volume remains constant. Charles's law describes how the volume of a gas increases as temperature increases at constant pressure. Gay-Lussac's law relates pressure and temperature at constant volume. The combined gas law incorporates these relationships. The ideal gas law (PV=nRT) relates pressure, volume, amount of gas, and temperature. Real gases deviate from ideal behavior at low temperatures or high pressures due to intermolecular forces and molecular size.Adv chem chapt 5
Adv chem chapt 5bobcatchemistry
╠²
The document discusses key concepts from gas chemistry including the composition and properties of the atmosphere, gas laws such as Boyle's, Charles', and Avogadro's laws, kinetic molecular theory, and concepts such as molar volume, partial pressures, and gas stoichiometry. It provides examples of calculations using the ideal gas law to determine quantities such as moles of gas, volume, pressure, and temperature changes.Ad
More from UC Berkeley Extension (20)
110 module1 2
110 module1 2UC Berkeley Extension
╠²
The document discusses scientific notation and its use in representing very large and very small numbers. Scientific notation expresses numbers as a decimal between 1 and 10 multiplied by a power of 10. For example, 1 million can be written as 1 x 106 and 0.0000001 can be written as 1 x 10-7. The document provides examples of using scientific notation and discusses how it is used on scientific calculators.Chem unit9
Chem unit9UC Berkeley Extension
╠²
The document discusses various properties of acids and bases, including that acids are sour and donate protons while bases are bitter and accept protons. It also discusses conjugate acid-base pairs, water's ability to act as an acid or base through self-ionization, and the pH scale. Key concepts covered include Br├Ėnsted-Lowry definitions of acids and bases, conjugate acid-base pairs, calculating pH and pOH, and neutralization reactions between acids and bases.Chem unit8
Chem unit8UC Berkeley Extension
╠²
The document discusses various concepts related to solutions and chemical equilibria including:
1) Types of solutions and units of concentration like molarity and ppm.
2) Factors that affect solubility, boiling point, and freezing point when a solute is added.
3) Equilibrium constants and how they relate concentrations of reactants and products.
4) Le Chatelier's principle which states that systems at equilibrium will respond to stresses like changes in concentration, pressure, or temperature to reduce the stress.E S A Unit6
E S A Unit6UC Berkeley Extension
╠²
Water is essential for life and has unique properties that make it suitable as a universal solvent and coolant. It exists in three phases - solid, liquid, and gas - and the water cycle circulates water between these phases, providing fresh water for living things through processes like evaporation and precipitation. However, water pollution from various sources threatens water quality and availability. Sustainable practices are needed to conserve water resources and ensure there is clean water to support the growing human population and global food supply.Chem Unit6
Chem Unit6UC Berkeley Extension
╠²
Stoichiometry is the quantitative relationship between reactants and products in chemical reactions. Hess's law states that the enthalpy change of a reaction is independent of the pathway and depends only on the initial and final states. Using Hess's law, one can determine the enthalpy change of a reaction by adding the enthalpy changes of multiple steps that combine to give the overall reaction. The standard enthalpy of formation of a compound is the enthalpy change when one mole of the substance is formed from its elements in their standard states.Esa Unit5
Esa Unit5UC Berkeley Extension
╠²
The document discusses several alternative energy sources including hydroelectric power, nuclear energy, solar energy, wind energy, and geothermal energy. Hydroelectric power harnesses the kinetic energy of falling or flowing water using dams, and provides around 7% of US energy needs. Nuclear energy currently supplies 17% of the world's electricity through fission in plants, but safety is a concern due to past incidents. Solar energy can be captured passively through building orientation or actively through photovoltaic cells, though efficiency and availability are issues. Wind energy uses turbines to convert kinetic energy of wind into electricity and capacity has been growing, though output is variable. Geothermal energy taps into heat from the Earth's interior through steam or hotChem Unit5
Chem Unit5UC Berkeley Extension
╠²
The document discusses various chemistry concepts including:
1. Avogadro's number defines the number of particles in one mole of a substance as 6.02x1023.
2. Molar mass is the mass of one mole of a pure substance and is used to convert between grams and moles.
3. Empirical and molecular formulas can be determined from percent composition data using mole ratios and molar mass.
4. Chemical equations represent chemical reactions and must be balanced to satisfy the law of conservation of mass.Esa Unit4
Esa Unit4UC Berkeley Extension
╠²
The document discusses the history of energy sources from the discovery of fire to modern times. It covers early sources like wood and biomass, as well as fossil fuels including coal, petroleum and natural gas. It then discusses issues with fossil fuel usage and alternatives being explored such as ethanol, hydrogen fuel cells, nuclear power, and renewable sources like solar, wind and hydroelectric. Newer technologies aim to reduce pollution and transition away from non-renewable energy sources.Chem Unit4
Chem Unit4UC Berkeley Extension
╠²
This document provides information about ions and salts, including:
- Cations are atoms that lose electrons to form positively charged ions, while anions are atoms that gain electrons to form negatively charged ions. Common examples like NaCl are described.
- Transition metal ions and polyatomic ions that can combine to form various salts are listed, along with methods for naming monoatomic and polyatomic salts.
- Properties of salts like high melting points and conductivity are discussed briefly.
- The basics of Lewis dot structures and molecular geometry are introduced for covalent bonding in organic compounds. Electronegativity and molecular polarity are also covered.Esa Unit3
Esa Unit3UC Berkeley Extension
╠²
The USDA conducted a culling program in Griggstown, New Jersey where they used pesticides to kill an estimated 5,000 starlings. Many local residents were shocked to discover dead starlings littering the roads and lawns in their community in the days following the culling. While the USDA notified local authorities of the program, they did not inform residents that dead birds could fall from the sky. Some residents remained concerned about the unusual event and unexpected presence of large numbers of dead starlings in the area.Chem Unit3
Chem Unit3UC Berkeley Extension
╠²
The document provides a timeline of the discovery of chemical elements from antiquity to the present day. It describes key developments in the understanding of periodic trends, including Dobereiner's triads, de Chantcourtois' spirals, Newlands' octaves, and Mendeleev's work that led to the formulation of the periodic table. The periodic table is then explained in more detail, covering trends in atomic structure, properties, and size across the families of elements.Chem Unit2
Chem Unit2UC Berkeley Extension
╠²
This document discusses the history of atomic theory from ancient Greek philosophers to modern atomic structure. It covers key discoveries such as the law of definite proportions, Dalton's atomic theory, the discovery of the electron, Rutherford's gold foil experiment, discovery of the neutron, and development of quantum numbers to describe electron configuration. Modern atomic structure is represented using electron shells, orbitals, and quantum numbers to explain periodic trends and chemical bonding.Chem Unit1
Chem Unit1UC Berkeley Extension
╠²
This document discusses various topics related to chemistry including units of measurement in the International System of Units (SI), properties of matter such as elements and compounds, physical and chemical changes, energy, heat transfer, temperature scales, specific heat capacity, and accuracy and precision in measurements. It provides definitions and examples for these fundamental chemistry concepts.Esa Unit1
Esa Unit1UC Berkeley Extension
╠²
1. The document describes two scientific experiments.
2. The first experiment found that chickens fed polished rice lacking vitamin B1 got sick, suggesting vitamin deficiency causes beriberi disease in humans.
3. The second experiment found that a mold called penicillin killed bacteria in culture dishes, leading Fleming to discover penicillin's ability to kill bacteria.Esa Unit2
Esa Unit2UC Berkeley Extension
╠²
This document summarizes key concepts relating to biomes and biodiversity, including the major terrestrial and aquatic biomes defined by temperature and precipitation patterns, the species found in each biome and their adaptations, ecological interactions like predation and mutualism, factors driving extinction, and examples of invasive species issues.ESAUnit2.ppt
ESAUnit2.pptUC Berkeley Extension
╠²
This document discusses key concepts in physics related to motion, forces, energy, and sound. It covers Aristotle and Galileo's early contributions, followed by Newton's laws of motion and universal law of gravitation. Key topics include linear and circular motion, forces, work, energy, heat transfer, and properties of sound waves like frequency, wavelength, and the Doppler effect. Equations for acceleration, velocity, forces, energy, heat, and sound are provided alongside examples to illustrate their application.ERMslides4
ERMslides4UC Berkeley Extension
╠²
1. The document discusses major climate events throughout history including the Dust Bowl of the 1930s and droughts in places like California, the Midwest, and along the Mississippi River.
2. It also covers evidence that an asteroid impact was the cause of the mass extinction that killed off the dinosaurs 65 million years ago, such as the iridium anomaly and shocked quartz found in the geologic record from that time.
3. The threat of future asteroid impacts is also discussed, along with efforts to detect potentially hazardous near-Earth asteroids and the possibility of deflecting them to avoid future impacts like the one that contributed to the KT extinction.Histslides4
Histslides4UC Berkeley Extension
╠²
This document provides a summary of key developments in science from alchemy to the beginnings of modern chemistry, physics, and medicine. Some of the major topics and figures discussed include the development of tools like the thermometer, discoveries about gases by Black, Priestley, and Lavoisier, Dalton's atomic theory, discoveries in electromagnetism by Faraday and Maxwell, germ theory and advances in medicine by Pasteur and Lister, Darwin's theory of evolution by natural selection, Mendel's laws of heredity, discoveries of DNA and the structure of the atom, and Einstein's theories of relativity.Uslides4
Uslides4UC Berkeley Extension
╠²
The document summarizes key concepts in chemistry and physics including:
1) The planetary model of the atom including electrons orbiting the nucleus in shells and orbitals.
2) The periodic table organized elements based on their chemical and physical properties including valence electrons.
3) Bonding between atoms including ionic bonding through the transfer of electrons and covalent bonding through the sharing of electron pairs.
4) The three states of matter - gases, liquids, and solids - and the forces between atoms in each state.ERMslides2
ERMslides2UC Berkeley Extension
╠²
The document discusses the history of plagues and pandemics throughout human history including the Plague of Justinian, the Black Death, influenza pandemics such as the 1918 Spanish Flu, emerging diseases like SARS, Ebola, HIV/AIDS, antibiotic resistance, and the threat of bioterrorism and biological weapons development. Key events, diseases, and historical figures are mentioned along with the biology of pathogens such as Yersinia pestis, influenza virus, anthrax, and smallpox. The complex transmission cycles between humans, animals, and environmental factors that drive disease emergence are also addressed.Ad
Chem Unit7
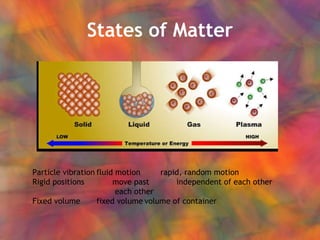
- 1. States of Matter Particle vibration fluid motion rapid, random motion Rigid positions move past independent of each other each other Fixed volume fixed volume volume of container
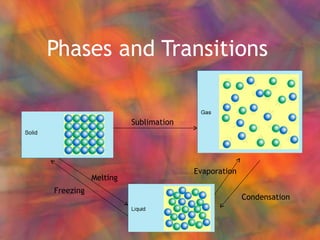
- 2. Phases and Transitions Sublimation Condensation Evaporation Melting Freezing
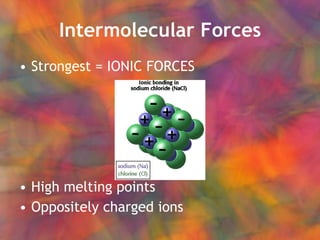
- 3. Intermolecular Forces Strongest = IONIC FORCES High melting points Oppositely charged ions
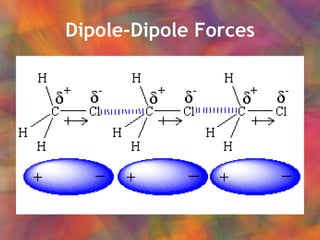
- 4. Dipole-Dipole Forces Dipole: contains both positively and negatively charged regions:
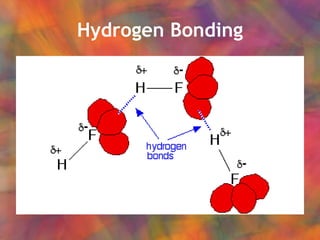
- 5. Hydrogen Bonding Special case of dipole-dipole forces Causes water to have higher than expected boiling point
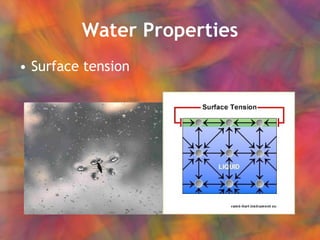
- 6. Water Properties Surface tension
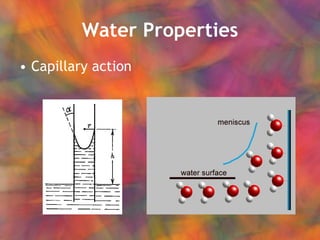
- 7. Water Properties Capillary action
- 8. Boiling Points Molecule Boiling Point H 2 O 100┬░C H 2 S ŌĆō 60.7 ┬░C H 2 Se ŌĆō 41┬░C H 2 Te ŌĆō 2┬░C
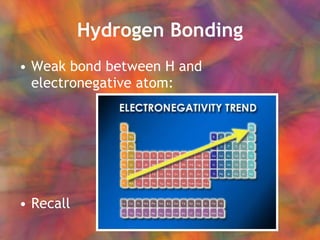
- 9. Hydrogen Bonding Weak bond between H and electronegative atom: Recall
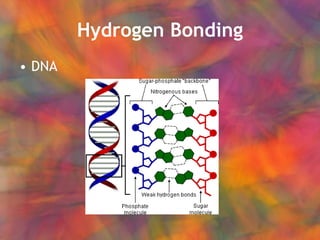
- 10. Hydrogen Bonding
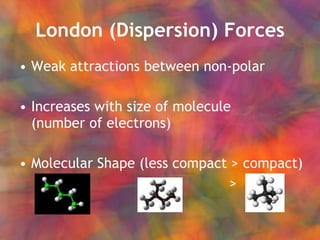
- 13. London (Dispersion) Forces Weak attractions between non-polar Increases with size of molecule (number of electrons) Molecular Shape (less compact > compact) > >
- 14. London (Dispersion) Forces Weak attractions between non-polar ŌĆ£ TemporaryŌĆØ or instantaneous dipoles
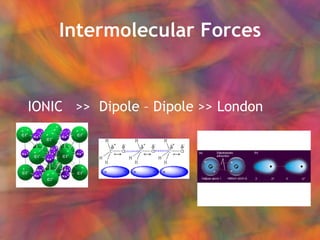
- 15. Intermolecular Forces IONIC >> Dipole ŌĆō Dipole >> London
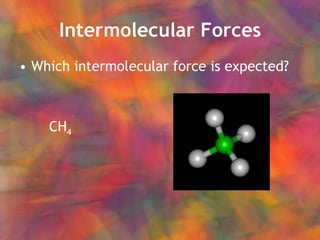
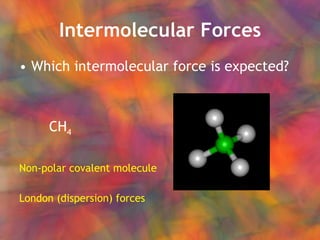
- 16. Intermolecular Forces Which intermolecular force is expected? CH 4
- 17. Intermolecular Forces Which intermolecular force is expected? CH 4 Non-polar covalent molecule London (dispersion) forces
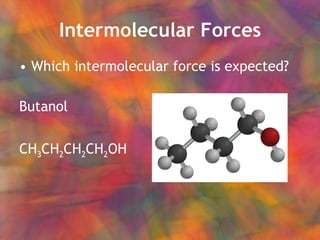
- 18. Intermolecular Forces Which intermolecular force is expected? Butanol CH 3 CH 2 CH 2 CH 2 OH
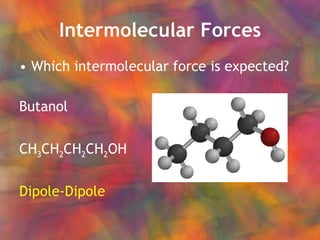
- 19. Intermolecular Forces Which intermolecular force is expected? Butanol CH 3 CH 2 CH 2 CH 2 OH Dipole-Dipole
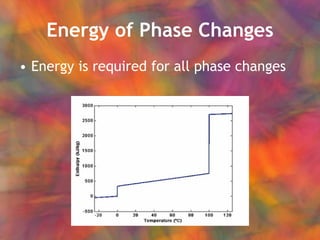
- 20. Energy of Phase Changes Energy is required for all phase changes
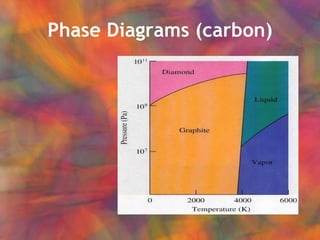
- 21. Phase Diagram (H 2 O)
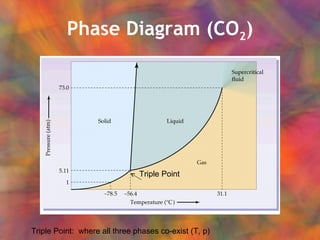
- 22. Phase Diagram (CO 2 ) Triple Point Triple Point: where all three phases co-exist (T, p)
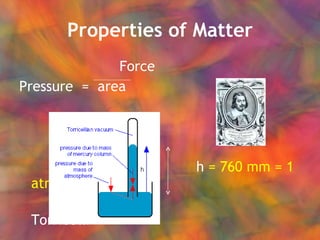
- 24. Properties of Matter Force Pressure = area h = 760 mm = 1 atm Torricelli barometer
- 25. Robert Boyle
- 26. BoyleŌĆÖs Law For a gas at constant T and n V and p are inversely proportional pV = constant
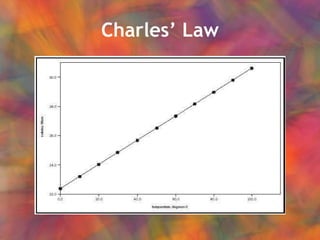
- 27. CharlesŌĆÖ Law At constant pressure, V/T = constant
- 28. CharlesŌĆÖ Law
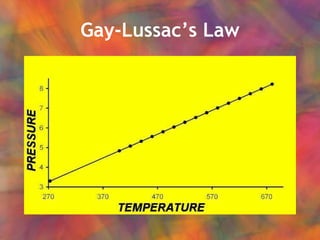
- 29. Gay ŌĆō LussacŌĆÖs Law In a constant volume: P/T = constant
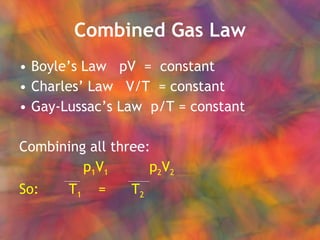
- 31. Combined Gas Law BoyleŌĆÖs Law pV = constant CharlesŌĆÖ Law V/T = constant Gay-LussacŌĆÖs Law p/T = constant Combining all three: p 1 V 1 p 2 V 2 So: T 1 = T 2
- 32. Combined Gas Law A sample of gas has a volume of 400 liters when its temperature is 20┬░C and its pressure is 300 mm Hg. What volume will the gas occupy at STP?
- 33. Combined Gas Law p 1 V 1 p 2 V 2 T 1 = T 2 T in Kelvin (300/760 mm Hg)(400 L) = (760 mm Hg) (V 2 ) (293 K) (273 K) V 2 = 147 Liters
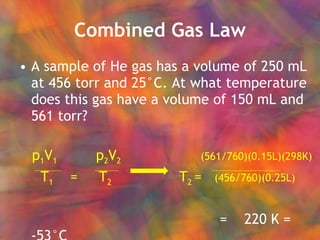
- 34. Combined Gas Law A sample of He gas has a volume of 250 mL at 456 torr and 25┬░C. At what temperature does this gas have a volume of 150 mL and 561 torr? p 1 V 1 p 2 V 2 T 1 = T 2
- 35. Combined Gas Law A sample of He gas has a volume of 250 mL at 456 torr and 25┬░C. At what temperature does this gas have a volume of 150 mL and 561 torr? p 1 V 1 p 2 V 2 T 2 = p 2 V 2 T 1 T 1 = T 2 p 1 V 1
- 36. Combined Gas Law A sample of He gas has a volume of 250 mL at 456 torr and 25┬░C. At what temperature does this gas have a volume of 150 mL and 561 torr? p 1 V 1 p 2 V 2 (561/760)(0.15L)(298K) T 1 = T 2 T 2 = (456/760)(0.25L) = 220 K = -53┬░C
- 37. Ideal Gases Non-interacting Point particles Randomly moving with elastic collisions (no energy lost)
- 38. Ideal Gases AvogadroŌĆÖs Law: Equal volumes of gas contain the same number of molecules at the same T & p. n = number of moles p 1 V 1 = constant n T 1
- 39. Ideal Gas Law p 1 V 1 = constant = 0.082 L atm = R n T 1 K mole = Universal Gas Constant One mole of gas at STP : Volume = nRT/p = (1 mole)(0.082 Latm)(273K)/1 atm = 22.4 Liters
- 40. Ideal Gas Law pV = nRT How many moles of Helium are present in a balloon that has a volume of 65 L at 20┬░ C and 705 torr? Given Needed V, T, p, R n n = pV/RT
- 41. Ideal Gas Law pV = nRT How many moles of Helium are present in a balloon that has a volume of 65 L at 20┬░ C and 705 torr? n = pV/RT = (705/760 atm)(65 L) (0.082 Latm/K)(293) = 2.5 moles He
- 42. Ideal Gas 6.2 liters of an ideal gas are contained at 3.0 atm and 37 ┬░C. How many moles of this gas are present?
- 43. Ideal Gas 6.2 liters of an ideal gas are contained at 3.0 atm and 37 ┬░C. How many moles of this gas are present? n = pV/RT = (3 atm)(6.2 L) (0.082 L atm/mole K ) (310 K) = 0.73 moles
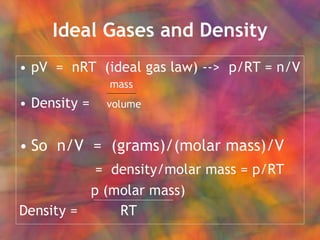
- 44. Ideal Gases and Density
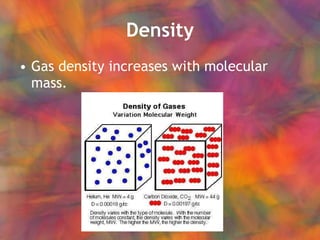
- 45. Density Gas density increases with molecular mass.
- 46. Density What is the density of NO 2 gas at 0.97 atm and 35┬░C? MW = 46 g/mole Molar mass p (46 g/mole)(0.97 atm) Density = RT (0.082 L atm/mole K) (308 K) = 1.767 g/L
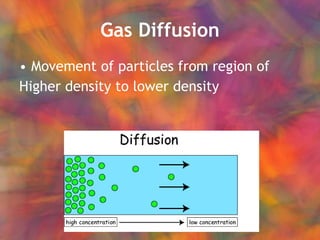
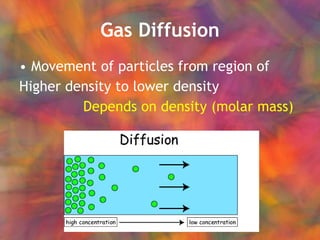
- 47. Gas Diffusion Movement of particles from region of Higher density to lower density
- 48. Gas Diffusion Movement of particles from region of Higher density to lower density Depends on density (molar mass)
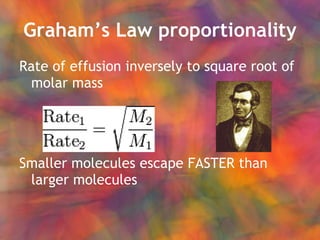
- 49. GrahamŌĆÖs Law proportionality Rate of effusion inversely to square root of molar mass Smaller molecules escape FASTER than larger molecules
- 50. DaltonŌĆÖs Law PT = P1 + P2 + P3 + . . . . Total pressure of a gas sample is the sun of the partial pressures.
- 51. DaltonŌĆÖs Law A mixture of O 2 , CO 2 and N 2 has a p T of 0.97 atm; if p O2 = 0.7 atm and p N2 = 0.12 atm, what is p CO2 ?
- 52. DaltonŌĆÖs Law A mixture of O 2 , CO 2 and N 2 has a p T of 0.97 atm; if p O2 = 0.7 atm and p N2 = 0.12 atm, what is p CO2 ? pT = p O2 + p N2 + p CO2 = 0.97 atm p CO2 = 0.97 atm - (0.7 atm + 0.12 atm) = 0.15 atm
- 53. DaltonŌĆÖs Law The partial pressures of CH 4 and O 2 are 0.175 atm and 0.25 atm. At 65┬░C in a volume of 2 L, how many moles of each gas are present?
- 54. DaltonŌĆÖs Law The partial pressures of CH 4 and O 2 are 0.175 atm and 0.25 atm. At 65┬░C in a volume of 2 L, how many moles of each gas are present? n CH4 = pV/RT = 0.175 atm (2L)/0.082 L atm/mole K (338 K = 0.126 moles n O2 = pV/RT = 0.25 atm (2L)/ 0.082 L atm/mole K (338 K) = 0.018 moles
- 55. Unit 7 Review Phases of Matter and Transitions Intermolecular Forces Phase Diagrams BoyleŌĆÖs, CharlesŌĆÖ, Gay LussacŌĆÖs Laws Ideal Gas Law pV = nRT GrahamŌĆÖs Law of Diffusion Partial Pressure
