Chemical Bonding
- 1. CHEMICAL BONDING Prepared by: Muhammed Danish Saleem
- 2. CHEMICAL BONDING Define as The attractive force that acts between two or more atoms to hold them together as a stable molecule.
- 3. Why chemical bonding arises? Every element want to get stability of noble gases. Every element wants to complete its duplet.
- 4. TYPES OF CHEMICAL BONDING There are three types of chemical bonding Ionic bond Covalent bond Co-ordinate covalent bond
- 5. IONIC BOND Those bond which are formed by the transference of an electrons. Example : NaCl
- 6. COVALENT BONDS The bond formed due to the mutual sharing of electrons between two atoms
- 7. TYPES OF COVALENT BOND There are three types of covalent bond Single covalent bond Double covalent bond Triple covalent bond
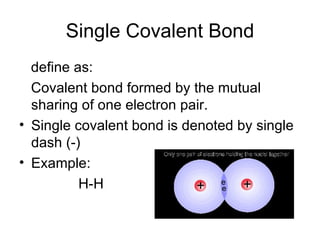
- 8. Single Covalent Bond define as: Covalent bond formed by the mutual sharing of one electron pair. Single covalent bond is denoted by single dash (-) Example: H-H
- 9. Double Covalent Bond Define as: Covalent bond formed by the mutual sharing of two electron pairs. Double covalent bond is denoted by double dash(=) Example: O=O
- 10. Triple Covalent Bond Covalent bond formed by the mutual sharing of three electron pairs. Triple covalent bond is denoted by three dash( ) Example H-Cl Cl-H
- 11. CO-ORDINATE COVALENT BOND Define as When both shared electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. Covalent bond in which the shared pair of electron is denoted by one atom only i.e the bond contain one electron pair donor and the other is electron acceptor. Co-ordinate covalent bond is denoted by an arrow ( -> ) towards the electron pair acceptor.
- 12. Formation of the Carbon Dioxide Molecule
- 13. Thank You