Chemical equations and reactions
- 2. CHEMICAL REACTIONS Reactants: Zn + I2 Product: Zn I2
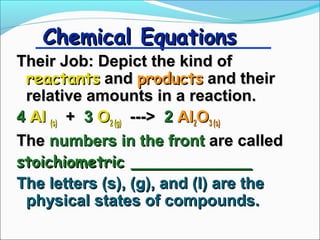
- 3. Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O2 (g) ---> 2 Al2O3 (s) The numbers in the front are called stoichiometric ____________ The letters (s), (g), and (l) are the physical states of compounds.
- 4. Symbols We Use ’éŚ+ ’éŚAdded to ’éŚŌåÆ ’éŚYields or Produces ’éŚ(s) ’éŚSubstance is a solid ’éŚ(l) ’éŚSubstance is a liquid ’éŚ(g) ’éŚSubstance is a gas ’éŚ(aq) ’éŚSubstance is aqueous ’éŚŌåö ’éŚReaction can go both ways (Reversible) ’éŚ╬ö ’éŚHeat if above arrows ’éŚŌåæ ’éŚSubs for (g) to show a gas is produced ’éŚŌåō ’éŚSubs for (s) to show a precipitate is formed
- 5. Introduction ’éŚChemical reactions occur when bonds between the outermost parts of atoms are formed or broken ’éŚChemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. ’éŚSymbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction
- 6. Parts of a Reaction Equation ’éŚChemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). ’éŚA + sign separates molecules on the same side ’éŚThe arrow is read as ŌĆ£yieldsŌĆØ ’éŚExample C + O2 ’āĀ CO2 ’éŚThis reads ŌĆ£carbon plus oxygen react to yield carbon dioxideŌĆØ
- 7. ’éŚThe charcoal used in a grill is basically carbon. The carbon reacts with oxygen to yield carbon dioxide. The chemical equation for this reaction, C + O2 ’āĀ CO2, contains the same information as the English sentence but has quantitative meaning as well.
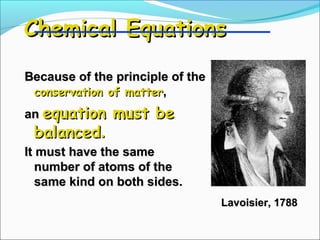
- 8. Chemical Equations Because of the principle of the conservation of matter, an equation must be balanced. It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788
- 9. Balancing Equations ’éŚWhen balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. ’éŚChanging the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)
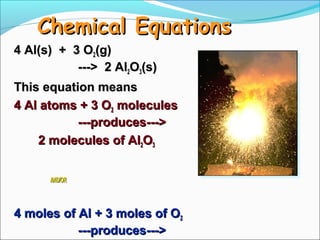
- 10. Chemical Equations 4 Al(s) + 3 O2(g) ---> 2 Al2O3(s) This equation means 4 Al atoms + 3 O2 molecules ---produces---> 2 molecules of Al2O3 AND/OR 4 moles of Al + 3 moles of O2 ---produces--->
- 11. Steps in Balancing An Equation There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: ’éŚ The numbers of atoms on both sides of the equation are now balanced.
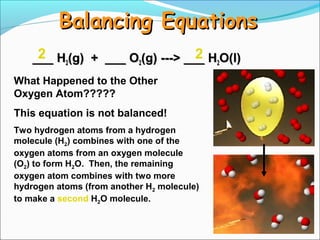
- 12. Balancing Equations 2 2 ___ H2(g) + ___ O2(g) ---> ___ H2O(l) What Happened to the Other Oxygen Atom????? This equation is not balanced! Two hydrogen atoms from a hydrogen molecule (H2) combines with one of the oxygen atoms from an oxygen molecule (O2) to form H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) to make a second H2O molecule.
- 13. Types of chemical equations COMBINATION DECOMPOSITION DISPLACEMENT DOUBLE-DISPLACEMENT
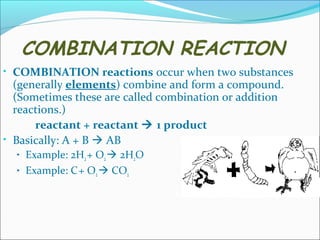
- 14. COMBINATION REACTION ŌĆó COMBINATION reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions.) reactant + reactant ’āĀ 1 product ŌĆó Basically: A + B ’āĀ AB ŌĆó Example: 2H2 + O2 ’āĀ 2H2O ŌĆó Example: C + O2 ’āĀ CO2
- 15. EXAMPLE OF A COMBINATION REACTION ŌĆó Here is another example of a synthesis reaction
- 16. DECOMPOSITION REACTION ŌĆó Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds ŌĆó 1 Reactant ’āĀ Product + Product ŌĆó In general: AB ’āĀ A + B ŌĆó Example: 2 H2O ’āĀ 2H2 + O2 ŌĆó Example: 2 HgO ’āĀ 2Hg + O2
- 17. EXAMPLE OF A DECOMPOSITION REACTION ŌĆó Another view of a decomposition reaction:
- 18. DISPLACEMENT REACTION ŌĆó Single Replacement Reactions occur when one element replaces another in a compound. ŌĆó A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). ŌĆó element + compound’āĀ element + compound A + BC ’āĀ AC + B (if A is a metal) OR A + BC ’āĀ BA + C (if A is a nonmetal) (remember the cation always goes first!) When H2O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!)
- 19. EXAMPLE OF A DISPLACEMENT REACTION ŌĆó Another view:
- 20. DOUBLE-DISPLACEMENT REACTION ŌĆó Double Replacement Reactions occur when a metal replaces a metal in a compound and a nonmetal replaces a nonmetal in a compound ŌĆó Compound + compound ’āĀ compound+ compound ŌĆó AB + CD ’āĀ AD + CB
- 21. For Spending your precious time