Chemical Structure: Chemical Bonding. Homonuclear Covalent Bonds
- 1. This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License HOMONUCLEAR COVALENT BONDS University of Lincoln presentation
- 2. Chemical Bonds A CHEMICAL BOND joins atoms together There are 4 types of chemical bond: COVALENT BONDS Ionic bonds Coordinate bonds Metallic bonds This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 3. Homonuclear Covalent Bonding What you need to knowŌĆ” Covalent bond formation Bond length Bond energy Bond order Relationship between bond length, bond energy and bond order Trends in the periodic table This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 4. DefinitionsŌĆ” A MOLECULE is a discrete neutral species resulting from the formation of a covalent bond or bonds between two or more atoms A HOMONUCLEAR BOND is a covalent bond between 2 identical atoms This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 5. Covalent Homonuclear Molecules This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Examples of covalent homonuclear molecules Hydrogen (H 2 ) Oxygen (O 2 ) Ozone (O 3 ) Iodine (I 2 ) Phosporous (P 4 ) Sulphur (S 6 ) Sulphur (S 8 )
- 6. Molecules with Homonuclear Bonds This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Molecules with one homonuclear bond Ethane (C 2 H 6 ) Hydrazine (N 2 H 4 ) Hydrogen peroxide (H 2 O 2 )
- 7. Making a Covalent Bond ŌĆō sharing valence electrons This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License In order to share valence electrons, 2 atoms have to come into close contact with each other 2 hydrogen atoms 1 hydrogen molecule, H 2 He 1 s 1 1 s 1 1 s 2 H H H H
- 8. Bringing 2 atoms together is not easy ŌĆō there are FOUR forces in playŌĆ” This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License + + ŌĆō ATOM A ATOM B
- 9. This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License The Four Forces Internuclear separation + + - - (2) (3) (4) (1)
- 10. How close do the atoms have to be to form a bond? The VAN DER WAALS RADIUS (r v ) of an atom X is measured as half of the distance of closest approach of 2 NON-BONDED atoms of X The COVALENT RADIUS (r cov ) of an atom X is taken as half of the internuclear distance (r) in a HOMONUCLEAR XŌĆōX bond. The internuclear distance (r) in a bonded pair of atoms is called the BOND LENGTH This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License r
- 11. Non-bonded vs Bonded Radii This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Note: the internuclear distance is SMALLER when atoms are bonded together 71 103 73 75 118 77 88 37 Covalent XŌĆōX radius (pm) BONDED 142 206 146 150 236 154 176 74 Covalent Bond Length (pm) (2 x r cov ) 135 F 185 S 140 O 154 N 210 Si 185 C 208 B 120 H Van der Waals radius (pm) NON-BONDED Element
- 12. ŌĆ” Hence, atoms must overlap to form a bond This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Non-bonded atoms ŌĆō NO OVERLAP of atomic orbitals Bonded atoms ŌĆō OVERLAP of atomic orbitals The bigger the overlap, the SHORTER the bond. The shorter the bond, the STRONGER it is. Bond length
- 13. Bond Energy Sometimes called the BOND ENTHALPY The BOND ENERGY is the amount of energy required to break a bond: This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License The larger the bond energy, the STRONGER the bond HŌĆōH 2H The bond energy is, therefore, a measure of how strong a bond is:
- 14. Breaking BondsŌĆ” This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Breaking the C-C bond produces two radicals Breaking the S-S bond opens up the ring structure C 2 H 6 S 6 2CH 3 ┬Ę ┬ĘS-S-S-S-S-S ┬Ę
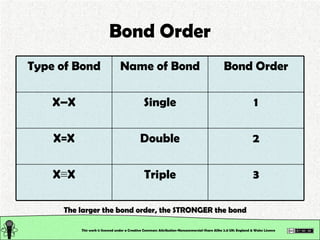
- 15. Bond Order This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License The larger the bond order, the STRONGER the bond 3 Triple X ŌēĪ X 2 Double X=X 1 Single XŌĆōX Bond Order Name of Bond Type of Bond
- 16. Some Bond Energies This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Group 17 IŌĆōI BrŌĆōBr ClŌĆōCl FŌĆōF S=S SŌĆōS O=O OŌĆōO Bond 490 P ŌēĪP 151 200 PŌĆōP 193 945 N ŌēĪ N 242 400 N=N 159 159 NŌĆōN 425 813 C ŌēĪ C 266 598 C=C 498 346 CŌĆōC 146 436 HŌĆōH Bond Energy (kJmol -1 ) Bond Energy (kJmol -1 ) Bond
- 17. Bond Energy & Bond Length This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License The shorter the bond, the higher the bond energy F is anomalous due to its small size. Bond energy would be expected to be ~275 kJmol-1 267 151 IŌĆōI 228 193 BrŌĆōBr 199 242 ClŌĆōCl 141 159 FŌĆōF Bond Length (pm) Bond Energy (kJmol -1 )
- 18. Adjacent Lone Pair Effect This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License veryclose Because F is a small atom (look at its position on the Periodic Table ŌĆō it is the smallest of the 1 st row elements) its valence electrons are very close and tend to repel each other. The two atoms are forced apart and the bond is weakened This anomalous behaviour is common in 1 st row elements, particularly, N, O and F F F
- 19. Group Trends in Homonuclear Single Bond Energies This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Note the anomalous behaviour of NŌĆōN, OŌĆōO and FŌĆōF. Group 14 show the expected trend
- 20. Formation of Multiple bonds This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Can only make a single bond Could make a double bond (sharing both of its unpaired electrons with another atom) Could make a double or a triple bond. A triple bond would be stronger (sharing all three unpaired electrons with another atom) N O F
- 21. Bond Energies for X 2 Molecules in Group 15 (in their natural state) This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License N 2 has very high bond energyŌĆ”why? Bond Energy (kJmol -1 )
- 22. Formation of the N 2 Molecule This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License N is small enough to overlap with another N atom sufficiently to share all three of its unpaired electrons and make a very strong TRIPLE BOND LINEAR Molecule N N N
- 23. Other elements in Group 15ŌĆ” P, As, Sb and Bi are TOO BIG to form multiple bonds ŌĆō they canŌĆÖt get close enough to overlap sufficiently These elements form SINGLE BONDS with three other atoms forming TETRAHEDRAL molecules This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 24. ŌĆ” Nitrogen forms a triple bond This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Other elements in Group 15 can only form single bonds X = N X = P, As, Sb or Bi
- 25. Periodic Trends in Bond Length, Bond Energy & Bond Order This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License X-X bond distances X-X bond dissociated enthalpy for X 2 molecules containing the first row elements
- 26. Bond Orders of the 1 st Row Elements This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License 1 FŌĆōF 2 O=O 3 N ŌēĪN 2 C=C 1 BŌĆōB Bond Order Homonuclear Diatomic
- 27. Summary This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 28. Things to RememberŌĆ” The covalent bond is formed by overlapping atomic orbitals This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 29. Bond Order (single, double, triple) Bond Energy (energy to break bond, kJmol -1 ) (measure of bond strength) Bond Length (internuclear distance, pm) Trends in the periodic tableŌĆ” This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 30. TRENDS: Bond energy increases as bond order increases Bond length decreases as bond order increases Bond energy decreases as bond length increases The shorter the bond, the stronger it is This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 31. Definitions Molecule Homonuclear bond van der Waals radius Covalent radius Bond length Bond energy Bond order This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License
- 32. This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License Acknowledgements JISC HEA Centre for Educational Research and Development School of natural and applied sciences School of Journalism SirenFM http:// tango.freedesktop.org