chemistrypresenation.pptx
Download as pptx, pdf0 likes38 views
The document discusses corrosion, including its causes, effects, and methods of prevention and control. Corrosion is the deterioration of metals due to chemical or electrochemical reactions with the environment. It can be caused by exposure to moisture, air, salts, acids, and other chemicals. Common forms of corrosion include uniform corrosion, pitting corrosion, and galvanic corrosion. Methods for preventing and controlling corrosion include protective coatings, cathodic protection, regular inspection and maintenance, and galvanizing. Effective corrosion control requires selecting appropriate methods based on the specific circumstances and type of corrosion present.
1 of 15
Download to read offline













Recommended
CORROSION.pptx
CORROSION.pptxPRAVEENKG20PHD0320
╠²
Corrosion is the deterioration of materials due to chemical reactions with their environment. It can occur due to factors like humidity, corrosive gases, stress, electrical currents, and bacteria. There are two main types of corrosion: dry/chemical corrosion which involves direct chemical reactions, and wet/electrochemical corrosion which involves the formation of anodic and cathodic areas on a metal surface. Common corrosion prevention methods include using surface coatings, galvanization, alloyed steels, cathodic protection, and new solutions like EonCoat which provides a maintenance-free protective layer.Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))
Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))Afzal Imam
╠²
Corrosion is the spontaneous disintegration of metals due to chemical or electrochemical reactions with the environment, causing the formation of compounds like oxides. It occurs as metals strive to return to stable states from higher energy levels, forming oxide films that can be protective or porous. Various methods exist to control corrosion, including proper design, material selection, environmental modifications, and the use of inhibitors.Corrosion and its preventive measures
Corrosion and its preventive measuresNagadatt Sharma Nagilla
╠²
The document discusses corrosion, its causes, types, and prevention methods, emphasizing its impact on metallic materials due to chemical and electrochemical actions. It outlines the corrosion cycle, principles, and variations such as pitting, stress, and crevice corrosion, along with factors influencing each type. Various prevention strategies are detailed, including the use of corrosion inhibitors, protective coatings, and careful material selection.corrosion.pptx
corrosion.pptxsofiagull2
╠²
Corrosion is a natural process that occurs when metals react with their environment, leading to deterioration over time. It is caused by chemical or electrochemical reactions between metals and substances like moisture, oxygen, acids, or salts. Corrosion can negatively impact appearance, strength, and integrity by causing rust, pitting, cracking, or loss of material thickness. The rate of corrosion is influenced by environmental conditions, the metal's composition and microstructure, and presence of corrosive substances, with some metals highly resistant and others highly prone. Methods to prevent and control corrosion include protective coatings, sacrificial anodes, corrosion inhibitors, cathodic protection, material selection, and regular inspection and maintenance.Engineering Chemistry_Corrosion.pptx
Engineering Chemistry_Corrosion.pptxHDaas1
╠²
This document provides an overview of the Engineering Chemistry course taught by Dr. Thathsara D. Maddumapatabandi. The course is worth 3 credits and assesses students through a midterm exam, quizzes, laboratory experiments, and a final exam. The course outline covers topics like atomic theory, chemical bonding, kinetics, equilibrium, thermodynamics, and corrosion. Corrosion is further discussed, defining it as the deterioration of metals due to reaction with the environment. Factors influencing corrosion and various protection methods are also summarized.corrosion
corrosionjagan vana
╠²
This document discusses corrosion, providing definitions and theories of corrosion. It describes dry corrosion theory involving direct reaction with gases like oxygen. Wet or galvanic corrosion theory involves formation of an electrochemical cell with oxidation at the anode and reduction at the cathode. The main types of corrosion discussed are uniform, pitting, intergranular, stress, crevice, and galvanic. Prevention methods covered include material selection, design considerations, environmental modification, and cathodic and anodic protection.Corrosion
CorrosionCSIR NAL
╠²
This document discusses corrosion, including its definition, mechanisms, rates, types, and ways to control it. Corrosion occurs via an electrochemical process where metal atoms dissolve into an electrolyte. The rate depends on factors like temperature, oxygen levels, and chemicals. Common types include uniform, galvanic, pitting and crevice corrosion. Control methods include protective coatings, inhibitors, material selection, cathodic protection and environmental control. Light alloys, steels and other metals used in various industries are susceptible to corrosion that must be addressed.Corrosion
CorrosionRhiza
╠²
There are 10 main types of corrosion described in the document:
1. Uniform corrosion which causes general dulling and roughening of surfaces.
2. Galvanic corrosion which occurs when two dissimilar metals contact in an electrolyte.
3. Stress corrosion which occurs in fabricated metals under mechanical stress.
4. The document also describes ways to prevent each type of corrosion, including using protective coatings, corrosion inhibitors, sacrificial anodes, and considering material selection and design modifications.Corrosion and its control
Corrosion and its controlvraju6
╠²
Corrosion is the decay of metals due to environmental factors like oxygen, moisture, and other gases. It can cause economic losses and safety issues like reduced machine lifespan and structural failures. There are two main types of corrosion - dry corrosion through direct chemical reactions and wet corrosion through electrochemical processes. Some factors that influence corrosion rates are the metal's position in the galvanic series, surface properties, environmental conditions, and purity. Common corrosion control methods include cathodic protection, protective coatings, and preventing exposure to corrosive substances.Corrosion Engineering, Problems, Causes, Remedy.pdf
Corrosion Engineering, Problems, Causes, Remedy.pdfAnnamalai Ram
╠²
Types of Corrosion, Theory of Corrosion, Causes, Methods to Control Corrosions, Materials and their chemical resistance tables, charts.Factors affecting corrosion & control measures
Factors affecting corrosion & control measuresSarala Prasanna Pattanaik
╠²
The document discusses the mechanisms, types, and factors influencing corrosion, including both electrochemical and chemical processes. It outlines various methods for protecting against corrosion, such as barrier, sacrificial, and cathodic protection, along with the use of coatings and alloys to enhance metal resistance. Additionally, it details the roles of environmental factors and metal purity in corrosion rates, along with specific inhibitors that can mitigate corrosion processes.Metal corrosion and its prevention
Metal corrosion and its preventionDr. Sandip Thorat
╠²
This document discusses metal corrosion and its prevention. It defines corrosion as the destruction or deterioration of solid metallic materials due to chemical or electrochemical attack from their environment. Corrosion is a major problem worldwide that impacts safety, health, and the environment. The document describes various types of corrosion including uniform corrosion, pitting corrosion, stress corrosion cracking, galvanic corrosion, and erosion corrosion. It also discusses factors that influence corrosion and various methods to prevent corrosion, such as using corrosion inhibitors, cathodic protection, coatings, and selecting corrosion-resistant materials.Forms of corrosion
Forms of corrosionchemnidhi
╠²
There are several types of corrosion that can occur:
1. Uniform and galvanic corrosion which results from direct chemical attack or from dissimilar metals in contact being exposed to an electrolyte.
2. Erosion corrosion caused by abrasive fluid flow removing a metal's protective surface film.
3. Crevice and pitting corrosion which are localized forms of corrosion occurring in cracks, crevices or defects in a metal surface.
4. Intergranular corrosion which attacks grain boundaries in metals or alloys.
5. Other forms include exfoliation, selective leaching, stress corrosion cracking, waterline corrosion affecting ship hulls, soil corrosion depending on soil conditions, and microbiologically influenced corrosion caused byCorrosion sb
Corrosion sbMirza Salman Baig
╠²
This document discusses corrosion, which is the process of a metal reacting with its environment and deteriorating over time. It provides three main theories of corrosion: dry corrosion, wet corrosion, and electrochemical corrosion. Dry corrosion involves direct chemical reaction with gases, while wet corrosion occurs in conducting liquids and involves the formation of galvanic cells. There are different types of corrosion like uniform, pitting, stress, and galvanic corrosion. Prevention methods include material selection, design considerations, environmental control, cathodic protection, anodic protection, and coatings. Cathodic protection makes the metal a cathode to reduce corrosion, while anodic protection forms a protective oxide layer.Steel corrosion
Steel corrosionAhmad Ali Miftah
╠²
The document discusses steel corrosion, defining it as the deterioration of a material due to environmental reactions, with major causes including the nature of the metal and its environment. It categorizes corrosion into various forms, such as wet and dry corrosion, and emphasizes the importance of corrosion prevention and monitoring methods like non-destructive testing. Additionally, it highlights the impacts of preventive measures on human and environmental health.metal corrosion and its prevention.pptx
metal corrosion and its prevention.pptxPankajSharma446574
╠²
Corrosion is the deterioration of metals due to chemical or electrochemical reaction with their environment. It is a major problem worldwide that impacts safety, health, and the environment. There are many types of corrosion including uniform corrosion, pitting corrosion, stress corrosion, galvanic corrosion, and erosion corrosion. Corrosion can be prevented through various methods like using corrosion inhibitors, coatings like painting, and selecting corrosion resistant materials. The document discusses the causes, mechanisms, types, and prevention methods of corrosion in detail.Corrosion, its causes and consequences and different control strategies
Corrosion, its causes and consequences and different control strategiesBittu Gupta
╠²
The document discusses corrosion, detailing its mechanisms, types (chemical and electrochemical), and factors affecting rates. It explains how metals deteriorate due to reactions with their environment, factors influencing corrosion, and methods for its control, including material selection and protective techniques like cathodic protection. Additionally, it highlights specific corrosion types like pitting and galvanic corrosion, alongside strategies for minimizing damage through proper design and inhibitors.Jcudydufufufudjfufududjfjfhdhxjcjcufufjfu
JcudydufufufudjfufududjfjfhdhxjcjcufufjfuEricColubanAlipan
╠²
The document discusses corrosion as the deterioration of materials due to environmental reactions, emphasizing its prevention through methods such as surface coatings and galvanization. It highlights the electrochemical nature of corrosion in aqueous environments, detailing anodic and cathodic processes and specific types of corrosion, including pitting and galvanic corrosion. Additionally, it describes methods for determining corrosion rates and offers insights into concentration cell corrosion and prevention strategies.CORROSION.ppt..............,...............
CORROSION.ppt..............,...............PrajwalKR21
╠²
The document provides a comprehensive overview of corrosion, detailing its causes, effects, and various types including electrochemical corrosion. It explains the detrimental impact of corrosion on metals, including loss of properties, reduced machinery lifespan, and significant economic costs estimated at 2 to 2.5 billion dollars annually. The document also discusses theories of corrosion, factors affecting it, and methods for corrosion control such as cathodic protection and surface coatings.Chapter 5: Corrosion & Non-ferrous Metal
Chapter 5: Corrosion & Non-ferrous Metalsyar 2604
╠²
Chapter 5 discusses corrosion and its impact on non-ferrous metals, explaining the mechanisms behind corrosion, such as dry and wet forms, and factors influencing corrosion rates like temperature and environment. It outlines various types of corrosion, including uniform, pitting, crevice, and stress corrosion, and emphasizes the importance of prevention techniques such as cathodic protection, material selection, protective coatings, and design considerations. Additionally, the chapter introduces non-ferrous metals like aluminum and copper, highlighting their properties and applications in industries.Chapter5 150109005402-conversion-gate02
Chapter5 150109005402-conversion-gate02Cleophas Rwemera
╠²
Corrosion occurs via electrochemical reactions between a material, usually a metal, and its environment. There are several types of corrosion including uniform corrosion, pitting, crevice corrosion, and intergranular corrosion. Corrosion can be prevented through methods like cathodic protection, selecting corrosion-resistant materials, using protective coatings, designing to avoid corrosion-prone situations, and alloying metals to enhance corrosion resistance. Managing corrosion is important as it can lead to infrastructure and equipment failures which are costly to repair and can impact safety.Mechanism Of Dry Corrosion
Mechanism Of Dry CorrosionAkash Chaturvedi
╠²
Dry corrosion occurs through direct chemical reaction between atmospheric gases like carbon dioxide and sulfur dioxide with metal surfaces in the absence of moisture. There are three main types of dry corrosion: 1) Oxidation corrosion, which occurs through direct reaction of oxygen with metals at ordinary temperatures in the absence of moisture. 2) Liquid metal corrosion, which occurs when a liquid metal flows over a solid metal at high temperatures. 3) Corrosion by other gases, where certain gases chemically react with metals to form protective or non-protective surface layers. The extent of dry corrosion depends on the environment and the nature and properties of the oxide film formed on the metal surface.Corrosion
CorrosionAbhijit Panchmatiya
╠²
The document discusses corrosion, which is the gradual destruction of metals through chemical or electrochemical reaction with the environment. Rusting of iron is a common example. There are two main types of corrosion - direct chemical corrosion which occurs through reaction with gases, and electrochemical corrosion which occurs when a metal is in contact with a conducting liquid. Electrochemical corrosion results from the formation of galvanic cells and the flow of current between anodic and cathodic areas. Methods of controlling corrosion include selecting corrosion-resistant materials, using protective coatings like paints and anodizing, adding corrosion inhibitors, and cathodic protection techniques.Factors affecting and prevention_Corrosion (1).ppt
Factors affecting and prevention_Corrosion (1).pptToranSahu18
╠²
The document discusses various factors influencing corrosion, including the nature of metals and their environments, such as purity, physical state, temperature, humidity, pH, and electrolyte nature. It examines mechanisms of corrosion and protective measures like barrier protection, sacrificial protection, alloy formation, and cathodic protection. Additionally, the document outlines methods to control and prevent corrosion, emphasizing the importance of material selection, equipment design, and use of inhibitors.CE336-09-Corrosion.PPT
CE336-09-Corrosion.PPTakramAwad2
╠²
The document discusses corrosion, which is the degradation of materials through chemical or electrochemical reaction with the environment. It describes the corrosion process, factors that influence corrosion, common forms of corrosion like uniform, galvanic, pitting and crevice corrosion. Methods to control and prevent corrosion include coatings, cathodic protection, and avoiding corrosive situations by selecting compatible metals and controlling environmental conditions. Reinforcing steel in concrete can corrode if the concrete lacks sufficient cover or has defects allowing moisture and chlorides to reach the steel.Corrosion
CorrosionGas & Oil Pakistan Ltd.
╠²
Group 6 presents information on corrosion, including definitions, types of corrosion, factors that affect corrosion rates, and methods for preventing corrosion. The group members are Waqas Ahmad, Umair Aslam, Tayyab Naveed, Muhammad Umair, and Muhammad Mudeser khalid. Corrosion is defined as the decay of metal due to chemical reactions with gases in the atmosphere. There are two main types of corrosion: dry corrosion caused by direct chemical reactions, and wet corrosion which is an electrochemical process. Factors that influence corrosion rates include the metal's position in the galvanic series, purity, physical state, and properties of the corrodent environment. Prevention methods consist of modifyingLecture 7_Corrosion and Control_copy.pdf
Lecture 7_Corrosion and Control_copy.pdfrazonclarence4
╠²
This document discusses corrosion of metals and its prevention. It defines corrosion and describes the electrochemical process. There are different types of corrosion like uniform corrosion, pitting corrosion, galvanic corrosion etc. Factors affecting corrosion rate are discussed. Common prevention methods mentioned are use of coatings like painting, galvanization; cathodic protection and use of corrosion inhibitors. Ceramic materials are resistant to corrosion due to their crystalline structure while polymers can degrade when exposed to solvents.IRJET- Corrosion Analysis by Acid Concentration in Oil and Gas Pipelines
IRJET- Corrosion Analysis by Acid Concentration in Oil and Gas PipelinesIRJET Journal
╠²
This document discusses corrosion in oil and gas pipelines. It identifies some key causes of corrosion as acid concentration, temperature, and presence of water and chemicals like CO2 and H2S. Internal corrosion is a major problem and can occur due to water, sediments, CO2, and H2S accumulating on the inner pipe surface. Corrosion causes damage through metal loss, weakening of pipe integrity, and environmental damage from oil spills. Proper material selection and corrosion protection methods are needed to control corrosion in pipelines and reduce economic and safety impacts.Structured Programming with C++ :: Kjell Backman
Structured Programming with C++ :: Kjell BackmanShabista Imam
╠²
Step into the world of high-performance programming with the Complete Guidance Book of C++ ProgrammingÔÇöa definitive resource for mastering one of the most powerful and versatile languages in computer science.
Whether you're a beginner looking to learn the fundamentals or an intermediate developer aiming to sharpen your skills, this book walks you through C++ from the ground up. You'll start with basics like variables, control structures, and functions, then progress to object-oriented programming (OOP), memory management, file handling, templates, and the Standard Template Library (STL).Introduction to Python Programming Language
Introduction to Python Programming Languagemerlinjohnsy
╠²
This PPT covers features, applications, variable, data types and statements in PythonMore Related Content
Similar to chemistrypresenation.pptx (20)
Corrosion and its control
Corrosion and its controlvraju6
╠²
Corrosion is the decay of metals due to environmental factors like oxygen, moisture, and other gases. It can cause economic losses and safety issues like reduced machine lifespan and structural failures. There are two main types of corrosion - dry corrosion through direct chemical reactions and wet corrosion through electrochemical processes. Some factors that influence corrosion rates are the metal's position in the galvanic series, surface properties, environmental conditions, and purity. Common corrosion control methods include cathodic protection, protective coatings, and preventing exposure to corrosive substances.Corrosion Engineering, Problems, Causes, Remedy.pdf
Corrosion Engineering, Problems, Causes, Remedy.pdfAnnamalai Ram
╠²
Types of Corrosion, Theory of Corrosion, Causes, Methods to Control Corrosions, Materials and their chemical resistance tables, charts.Factors affecting corrosion & control measures
Factors affecting corrosion & control measuresSarala Prasanna Pattanaik
╠²
The document discusses the mechanisms, types, and factors influencing corrosion, including both electrochemical and chemical processes. It outlines various methods for protecting against corrosion, such as barrier, sacrificial, and cathodic protection, along with the use of coatings and alloys to enhance metal resistance. Additionally, it details the roles of environmental factors and metal purity in corrosion rates, along with specific inhibitors that can mitigate corrosion processes.Metal corrosion and its prevention
Metal corrosion and its preventionDr. Sandip Thorat
╠²
This document discusses metal corrosion and its prevention. It defines corrosion as the destruction or deterioration of solid metallic materials due to chemical or electrochemical attack from their environment. Corrosion is a major problem worldwide that impacts safety, health, and the environment. The document describes various types of corrosion including uniform corrosion, pitting corrosion, stress corrosion cracking, galvanic corrosion, and erosion corrosion. It also discusses factors that influence corrosion and various methods to prevent corrosion, such as using corrosion inhibitors, cathodic protection, coatings, and selecting corrosion-resistant materials.Forms of corrosion
Forms of corrosionchemnidhi
╠²
There are several types of corrosion that can occur:
1. Uniform and galvanic corrosion which results from direct chemical attack or from dissimilar metals in contact being exposed to an electrolyte.
2. Erosion corrosion caused by abrasive fluid flow removing a metal's protective surface film.
3. Crevice and pitting corrosion which are localized forms of corrosion occurring in cracks, crevices or defects in a metal surface.
4. Intergranular corrosion which attacks grain boundaries in metals or alloys.
5. Other forms include exfoliation, selective leaching, stress corrosion cracking, waterline corrosion affecting ship hulls, soil corrosion depending on soil conditions, and microbiologically influenced corrosion caused byCorrosion sb
Corrosion sbMirza Salman Baig
╠²
This document discusses corrosion, which is the process of a metal reacting with its environment and deteriorating over time. It provides three main theories of corrosion: dry corrosion, wet corrosion, and electrochemical corrosion. Dry corrosion involves direct chemical reaction with gases, while wet corrosion occurs in conducting liquids and involves the formation of galvanic cells. There are different types of corrosion like uniform, pitting, stress, and galvanic corrosion. Prevention methods include material selection, design considerations, environmental control, cathodic protection, anodic protection, and coatings. Cathodic protection makes the metal a cathode to reduce corrosion, while anodic protection forms a protective oxide layer.Steel corrosion
Steel corrosionAhmad Ali Miftah
╠²
The document discusses steel corrosion, defining it as the deterioration of a material due to environmental reactions, with major causes including the nature of the metal and its environment. It categorizes corrosion into various forms, such as wet and dry corrosion, and emphasizes the importance of corrosion prevention and monitoring methods like non-destructive testing. Additionally, it highlights the impacts of preventive measures on human and environmental health.metal corrosion and its prevention.pptx
metal corrosion and its prevention.pptxPankajSharma446574
╠²
Corrosion is the deterioration of metals due to chemical or electrochemical reaction with their environment. It is a major problem worldwide that impacts safety, health, and the environment. There are many types of corrosion including uniform corrosion, pitting corrosion, stress corrosion, galvanic corrosion, and erosion corrosion. Corrosion can be prevented through various methods like using corrosion inhibitors, coatings like painting, and selecting corrosion resistant materials. The document discusses the causes, mechanisms, types, and prevention methods of corrosion in detail.Corrosion, its causes and consequences and different control strategies
Corrosion, its causes and consequences and different control strategiesBittu Gupta
╠²
The document discusses corrosion, detailing its mechanisms, types (chemical and electrochemical), and factors affecting rates. It explains how metals deteriorate due to reactions with their environment, factors influencing corrosion, and methods for its control, including material selection and protective techniques like cathodic protection. Additionally, it highlights specific corrosion types like pitting and galvanic corrosion, alongside strategies for minimizing damage through proper design and inhibitors.Jcudydufufufudjfufududjfjfhdhxjcjcufufjfu
JcudydufufufudjfufududjfjfhdhxjcjcufufjfuEricColubanAlipan
╠²
The document discusses corrosion as the deterioration of materials due to environmental reactions, emphasizing its prevention through methods such as surface coatings and galvanization. It highlights the electrochemical nature of corrosion in aqueous environments, detailing anodic and cathodic processes and specific types of corrosion, including pitting and galvanic corrosion. Additionally, it describes methods for determining corrosion rates and offers insights into concentration cell corrosion and prevention strategies.CORROSION.ppt..............,...............
CORROSION.ppt..............,...............PrajwalKR21
╠²
The document provides a comprehensive overview of corrosion, detailing its causes, effects, and various types including electrochemical corrosion. It explains the detrimental impact of corrosion on metals, including loss of properties, reduced machinery lifespan, and significant economic costs estimated at 2 to 2.5 billion dollars annually. The document also discusses theories of corrosion, factors affecting it, and methods for corrosion control such as cathodic protection and surface coatings.Chapter 5: Corrosion & Non-ferrous Metal
Chapter 5: Corrosion & Non-ferrous Metalsyar 2604
╠²
Chapter 5 discusses corrosion and its impact on non-ferrous metals, explaining the mechanisms behind corrosion, such as dry and wet forms, and factors influencing corrosion rates like temperature and environment. It outlines various types of corrosion, including uniform, pitting, crevice, and stress corrosion, and emphasizes the importance of prevention techniques such as cathodic protection, material selection, protective coatings, and design considerations. Additionally, the chapter introduces non-ferrous metals like aluminum and copper, highlighting their properties and applications in industries.Chapter5 150109005402-conversion-gate02
Chapter5 150109005402-conversion-gate02Cleophas Rwemera
╠²
Corrosion occurs via electrochemical reactions between a material, usually a metal, and its environment. There are several types of corrosion including uniform corrosion, pitting, crevice corrosion, and intergranular corrosion. Corrosion can be prevented through methods like cathodic protection, selecting corrosion-resistant materials, using protective coatings, designing to avoid corrosion-prone situations, and alloying metals to enhance corrosion resistance. Managing corrosion is important as it can lead to infrastructure and equipment failures which are costly to repair and can impact safety.Mechanism Of Dry Corrosion
Mechanism Of Dry CorrosionAkash Chaturvedi
╠²
Dry corrosion occurs through direct chemical reaction between atmospheric gases like carbon dioxide and sulfur dioxide with metal surfaces in the absence of moisture. There are three main types of dry corrosion: 1) Oxidation corrosion, which occurs through direct reaction of oxygen with metals at ordinary temperatures in the absence of moisture. 2) Liquid metal corrosion, which occurs when a liquid metal flows over a solid metal at high temperatures. 3) Corrosion by other gases, where certain gases chemically react with metals to form protective or non-protective surface layers. The extent of dry corrosion depends on the environment and the nature and properties of the oxide film formed on the metal surface.Corrosion
CorrosionAbhijit Panchmatiya
╠²
The document discusses corrosion, which is the gradual destruction of metals through chemical or electrochemical reaction with the environment. Rusting of iron is a common example. There are two main types of corrosion - direct chemical corrosion which occurs through reaction with gases, and electrochemical corrosion which occurs when a metal is in contact with a conducting liquid. Electrochemical corrosion results from the formation of galvanic cells and the flow of current between anodic and cathodic areas. Methods of controlling corrosion include selecting corrosion-resistant materials, using protective coatings like paints and anodizing, adding corrosion inhibitors, and cathodic protection techniques.Factors affecting and prevention_Corrosion (1).ppt
Factors affecting and prevention_Corrosion (1).pptToranSahu18
╠²
The document discusses various factors influencing corrosion, including the nature of metals and their environments, such as purity, physical state, temperature, humidity, pH, and electrolyte nature. It examines mechanisms of corrosion and protective measures like barrier protection, sacrificial protection, alloy formation, and cathodic protection. Additionally, the document outlines methods to control and prevent corrosion, emphasizing the importance of material selection, equipment design, and use of inhibitors.CE336-09-Corrosion.PPT
CE336-09-Corrosion.PPTakramAwad2
╠²
The document discusses corrosion, which is the degradation of materials through chemical or electrochemical reaction with the environment. It describes the corrosion process, factors that influence corrosion, common forms of corrosion like uniform, galvanic, pitting and crevice corrosion. Methods to control and prevent corrosion include coatings, cathodic protection, and avoiding corrosive situations by selecting compatible metals and controlling environmental conditions. Reinforcing steel in concrete can corrode if the concrete lacks sufficient cover or has defects allowing moisture and chlorides to reach the steel.Corrosion
CorrosionGas & Oil Pakistan Ltd.
╠²
Group 6 presents information on corrosion, including definitions, types of corrosion, factors that affect corrosion rates, and methods for preventing corrosion. The group members are Waqas Ahmad, Umair Aslam, Tayyab Naveed, Muhammad Umair, and Muhammad Mudeser khalid. Corrosion is defined as the decay of metal due to chemical reactions with gases in the atmosphere. There are two main types of corrosion: dry corrosion caused by direct chemical reactions, and wet corrosion which is an electrochemical process. Factors that influence corrosion rates include the metal's position in the galvanic series, purity, physical state, and properties of the corrodent environment. Prevention methods consist of modifyingLecture 7_Corrosion and Control_copy.pdf
Lecture 7_Corrosion and Control_copy.pdfrazonclarence4
╠²
This document discusses corrosion of metals and its prevention. It defines corrosion and describes the electrochemical process. There are different types of corrosion like uniform corrosion, pitting corrosion, galvanic corrosion etc. Factors affecting corrosion rate are discussed. Common prevention methods mentioned are use of coatings like painting, galvanization; cathodic protection and use of corrosion inhibitors. Ceramic materials are resistant to corrosion due to their crystalline structure while polymers can degrade when exposed to solvents.IRJET- Corrosion Analysis by Acid Concentration in Oil and Gas Pipelines
IRJET- Corrosion Analysis by Acid Concentration in Oil and Gas PipelinesIRJET Journal
╠²
This document discusses corrosion in oil and gas pipelines. It identifies some key causes of corrosion as acid concentration, temperature, and presence of water and chemicals like CO2 and H2S. Internal corrosion is a major problem and can occur due to water, sediments, CO2, and H2S accumulating on the inner pipe surface. Corrosion causes damage through metal loss, weakening of pipe integrity, and environmental damage from oil spills. Proper material selection and corrosion protection methods are needed to control corrosion in pipelines and reduce economic and safety impacts.Recently uploaded (20)
Structured Programming with C++ :: Kjell Backman
Structured Programming with C++ :: Kjell BackmanShabista Imam
╠²
Step into the world of high-performance programming with the Complete Guidance Book of C++ ProgrammingÔÇöa definitive resource for mastering one of the most powerful and versatile languages in computer science.
Whether you're a beginner looking to learn the fundamentals or an intermediate developer aiming to sharpen your skills, this book walks you through C++ from the ground up. You'll start with basics like variables, control structures, and functions, then progress to object-oriented programming (OOP), memory management, file handling, templates, and the Standard Template Library (STL).Introduction to Python Programming Language
Introduction to Python Programming Languagemerlinjohnsy
╠²
This PPT covers features, applications, variable, data types and statements in PythonProposal for folders structure division in projects.pdf
Proposal for folders structure division in projects.pdfMohamed Ahmed
╠²
Proposal for folders structure division in projectsStay Safe Women Security Android App Project Report.pdf
Stay Safe Women Security Android App Project Report.pdfKamal Acharya
╠²
WomenÔÇÖs security is a critical issue in todayÔÇÖs world and itÔÇÖs very much needed for every individual
to be acting over such an issue. This document describes a GPS based ÔÇ£Women Security System''
that provides the combination of GPS devices as well as provide alerts and messages with an
emergency button trigger whenever somebody is in trouble They might not have so much time, all
that they have to do is generate a distress emergency signal by shaking up their phone. Our system
provides a realizable, cost effective solution to problem detection. Nowdays due to recently
happened cases such as rape by drivers or colleagues, burglary etc., women security, especially
women security has become the foremost priority of the world. System uses the Global Positioning
System (GPS) technology to find out the location of women. The information of women's position
provided by the device can be viewed on Google maps using Internet or specialized software. The
companies are looking for-ward to the security problem and require a system that will efficiently
evaluate the problem of women security working in night shifts, traveling alone. We focus on the
proposed model that can be used to deal with the security issue of women using GPS based tracking
systems.Generative AI & Scientific Research : Catalyst for Innovation, Ethics & Impact
Generative AI & Scientific Research : Catalyst for Innovation, Ethics & ImpactAlqualsaDIResearchGr
╠²
Invited keynote at the Artificial Intelligence Symposium on AI-powered Research Innovation, taking place at ENSEM (L'├ëcole Nationale Sup├®rieure d'├ëlectricit├® et de M├®canique), Casablanca on June 21, 2025. IÔÇÖll be giving a keynote titled: "Generative AI & Scientific Research: Catalyst for Innovation, Ethics & Impact". Looking forward to engaging with researchers and doctoral students on how Generative AI is reshaping the future of science, from discovery to governance ÔÇö with both opportunities and responsibilities in focus.
#AI hashtag#GenerativeAI #ScientificResearch #Innovation #Ethics #Keynote #AIinScience #GAI #ResearchInnovation #Casablanca
1. Thinking, Creative Thinking, Innovation
2. Societies Evolution from 1.0 to 5.0
3. AI - 3P Approach, Use Cases & Innovation
4. GAI & Creativity
5. TrustWorthy AI
6. Guidelines on The Responsible use of GAI In ResearchDeep Learning for Image Processing on 16 June 2025 MITS.pptx
Deep Learning for Image Processing on 16 June 2025 MITS.pptxresming1
╠²
This covers how image processing or the field of computer vision has advanced with the advent of neural network architectures ranging from LeNet to Vision transformers. It covers how deep neural network architectures have developed step-by-step from the popular CNNs to ViTs. CNNs and its variants along with their features are described. Vision transformers are introduced and compared with CNNs. It also shows how an image is processed to be given as input to the vision transformer. It give the applications of computer vision.May 2025: Top 10 Read Articles in Data Mining & Knowledge Management Process
May 2025: Top 10 Read Articles in Data Mining & Knowledge Management ProcessIJDKP
╠²
Data mining and knowledge discovery in databases have been attracting a significant amount of research, industry, and media attention of late. There is an urgent need for a new generation of computational theories and tools to assist researchers in extracting useful information from the rapidly growing volumes of digital data.
This Journal provides a forum for researchers who address this issue and to present their work in a peer-reviewed open access forum. Authors are solicited to contribute to the Journal by submitting articles that illustrate research results, projects, surveying works and industrial experiences that describe significant advances in the following areas, but are not limited to these topics only.Introduction to Natural Language Processing - Stages in NLP Pipeline, Challen...
Introduction to Natural Language Processing - Stages in NLP Pipeline, Challen...resming1
╠²
Lecture delivered in 2021. This gives an introduction to Natural Language Processing. It describes the use cases of NLP in daily life. It discusses the stages in NLP Pipeline. It highlights the challenges involved covering the different levels of ambiguity that could arise. It also gives a brief note on the present scenario with the latest language models, tools and frameworks/libraries for NLP.Microwatt: Open Tiny Core, Big Possibilities
Microwatt: Open Tiny Core, Big PossibilitiesIBM
╠²
Microwatt is a lightweight, open-source core based on the OpenPOWER ISA.
ItÔÇÖs designed for FPGAs and easy experimentation in chip design.
Ideal for education, prototyping, and custom silicon development.
Fully open, it empowers developers to learn, modify, and innovate.Deep Learning for Natural Language Processing_FDP on 16 June 2025 MITS.pptx
Deep Learning for Natural Language Processing_FDP on 16 June 2025 MITS.pptxresming1
╠²
This gives an introduction to how NLP has evolved from the time of World War II till this date through the advances in approaches, architectures and word representations. From rule based approaches, it advanced to statistical approaches. from traditional machine learning algorithms it advanced to deep neural network architectures. Deep neural architectures include recurrent neural networks, long short term memory, gated recurrent units, seq2seq models, encoder decoder models, transformer architecture, upto large language models and vision language models which are multimodal in nature.Rapid Prototyping for XR: Lecture 2 - Low Fidelity Prototyping.
Rapid Prototyping for XR: Lecture 2 - Low Fidelity Prototyping.Mark Billinghurst
╠²
This is lecture 2 on the Rapid Prototyping for XR course taught by Mark Billingurst on June 10th 2025. This lecture is about Low Fidelity Prototyping.(Continuous Integration and Continuous Deployment/Delivery) is a fundamental ...
(Continuous Integration and Continuous Deployment/Delivery) is a fundamental ...ketan09101
╠²
(Continuous Integration and Continuous Deployment/Delivery) is a fundamental practice in DevOps that streamlines software development and deployment.Fatality due to Falls at Working at Height
Fatality due to Falls at Working at Heightssuserb8994f
╠²
It is related to fatality due to falls at working at heightGenerative AI & Scientific Research : Catalyst for Innovation, Ethics & Impact
Generative AI & Scientific Research : Catalyst for Innovation, Ethics & ImpactAlqualsaDIResearchGr
╠²
chemistrypresenation.pptx
- 1. CORROSION CONTROL METHODS, CAUSES AND EFFECTS OF CORROSION P. SAI CHARAN GOUD R. SANDEEP GOUD U. ASISH VARMA
- 2. WHAT IS CORROSION? Corrosion is the gradual deterioration of a material, usually a metal, due to chemical or electrochemical reactions with its environment. It often results in the loss of structural integrity and can lead to the failure of the material or the system it's a part of. Corrosion can be caused by a variety of factors, including exposure to moisture, air, salt, acids, and other chemicals. It can occur in various forms, such as uniform corrosion, pitting corrosion, galvanic corrosion, crevice corrosion, and stress corrosion cracking. Corrosion prevention techniques include the use of protective coatings, sacrificial anodes, cathodic protection, and proper material selection. Regular inspection and maintenance are also important to detect and prevent corrosion before it causes significant damage.
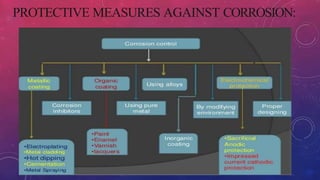
- 3. CORROSION CONTROL METHODS: . Protective coatings . Cathodic protection . Regular inspection and maintenance . Galvanizing
- 4. PROTECTIVE COATINGS: Applying a protective layer, such as paint or a coating, can prevent the metal from coming into contact with the environment and reduce the risk of corrosion. CATHODICPROTECTION:This method involves applying a direct electrical current to the metal, which can help to prevent corrosion. Cathodic protection can be achieved through sacrificialanodes, impressed current systems, or a combination of both.
- 5. REGULAR INSPECTIONAND MAINTENANCE: Regular inspection and maintenance can help to detect and prevent corrosion before it causes significant damage. This can include cleaning the surface of the metal, repairing damaged coatings, and replacing corroded components. GALVANIZING: This method involves coating the metal with a layer of zinc, which can protect the metal from corrosion. Zinc is more reactive than the metal it is protecting, so it corrodes in preference to the underlying metal.
- 6. NOTE: It's important to note that different corrosion control methods may be more or less effective depending on the specific circumstances and the type of corrosion that is occurring. A combination of methods may be necessary to effectively prevent or control corrosion.
- 7. CAUSES OF CORROSION: Metal corrodes when it reacts with another substance such as oxygen, hydrogen, an electricalcurrent or evendirtand bacteria. Corrosion can also happen when metals like steel are placed under too much stress causing the material to crack. CORROSIONCAN BE CAUSED BYA VARIETY OF FACTORS, INCLUDING: Exposure to moisture: Moisture, especially in the form of water, is one of the primary causes of corrosion. When metals are exposed to water or high humidity, they can begin to corrode. Exposure to air: Oxygen in the air can react with the metal and cause corrosion, especially in the presence of moisture.
- 8. Exposure to salt: Salt, such as in seawater or road salt, can be particularly corrosive to metals. Exposure to acids and other chemicals: Acids and other chemicals can react with the metal and cause corrosion. Galvanic reactions: when two dis- Similar metals are in contact, an electrochemical reaction can occur that leads to corrosion of one of the metals.
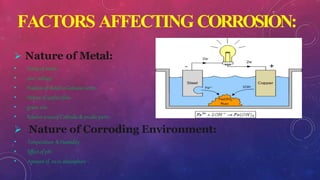
- 9. FACTORS AFFECTING CORROSION: ´âÿ Nature of Metal: ÔÇó Purityof metal. ÔÇó over voltage. ÔÇó Positionof metalin Galvanicseries. ÔÇó Nature of surfacefilm. ÔÇó grain size. ÔÇó Relativeareasof Cathodic& anodicparts. ´âÿ Nature of Corroding Environment: ÔÇó Temperature &Humidity . ÔÇó Effectof pH. ÔÇó Amountof 02inatmosphere .
- 10. NOTE: Overall, the negative effects of corrosion can be significant and costly. Implementing effective corrosion control methods can help to prevent or minimize these effects and extend the lifespan of metal components.
- 11. TYPES OFCORROSION: ´âÿ Dry or Chemical Corrosion ´âÿWet or Electrochemical Corrosion
- 12. CHEMICAL ( OR DRY ) CORROSION: Chemical corrosion is a type of corrosion that is caused by chemical reactions between a metal and its environment. This can occur when the metal is exposed to acids, bases, salts, or other chemicals. Chemical corrosion can take several different forms, including uniform corrosion, pitting corrosion, and intergranular corrosion . The chemical reactions that cause corrosion typically involve the transfer of electrons between the metal and the environment, leading to the formation of metal ions and the release of hydrogen gas. In some cases, the reaction can be accelerated by the presence of catalysts or other factors.
- 13. ELECTROCHEMICAL ( OR WET ) CORROSION: Electrochemical corrosion, also known as galvanic corrosion, is a type of corrosion that occurs when two different metals are in contact with each other in the presence of an electrolyte, such as saltwater or an acidic solution. The two metals form a galvanic cell, with one metal acting as the anode and the other metal acting as the cathode . The anode, which is the metal that is less noble, undergoes oxidation and corrodes, while the cathode, which is the more noble metal, is protected. The corrosion can occur at the interface between the two metals or at the surface of the anode.
- 14. PROTECTIVE MEASURES AGAINST CORROSION: