Classification of Chemical Reactions
7 likes6,450 views
Microsoft Power Point presentation used to teach the five types of chemical reactions to Chemistry I Honors
1 of 21
















Recommended
C2.1 structure and bonding



C2.1 structure and bondingSteve Bishop
╠²
Ionic compounds form giant lattice structures when oppositely charged ions bond via electrostatic forces. This ionic bonding results in high melting points and the ability to conduct electricity when molten or dissolved. Covalent bonds involve electron sharing and can form either simple molecules with weak intermolecular forces or giant covalent structures with very high melting points due to numerous strong covalent bonds. Metallic bonding involves delocalized electrons that act as glue between positive metal ions, allowing them to slide past one another.Types of Chemical Reactions



Types of Chemical ReactionsMelinda MacDonald
╠²
The document describes the main types of chemical reactions: synthesis reactions where two or more reactants form one product, decomposition reactions where one reactant breaks down into multiple products, replacement reactions including single replacement where one element replaces another in a compound and double replacement where ions switch between compounds, and combustion reactions where a substance reacts with oxygen releasing energy.Chemical reactions and equations



Chemical reactions and equationsAnushka Ninama
╠²
This document provides an overview of chemical reactions and equations. It discusses chemical changes and how they can be represented by balanced chemical equations. The main types of chemical reactions covered are combination reactions, decomposition reactions, displacement reactions, and oxidation-reduction reactions. Examples of each type of reaction are given. The document also explains how to balance chemical equations by ensuring equal numbers of each type of atom on both sides of the reaction equation.Chemical equations & reactions



Chemical equations & reactionsBibhuti Bhushan
╠²
The document discusses chemical equations and reactions. It defines key terms like reactants, products, and coefficients. It explains how to write and balance chemical equations. It also describes different types of chemical reactions like synthesis, decomposition, and single/double replacement reactions. Guidelines are provided for predicting products and writing balanced equations.Latent heat



Latent heatPadme Amidala
╠²
When energy is absorbed as heat by a solid or liquid, the temperature of the object does not necessarily rise.
The thermal energy may cause the mass to change from one phase, or state, to another.
The amount of energy per unit mass that must be transferred as heat when a mass undergoes a phase change is called the heat of transformation, L.
5.4 exothermic and endothermic reactions



5.4 exothermic and endothermic reactionsMartin Brown
╠²
This document discusses exothermic and endothermic reactions. Exothermic reactions release heat, while endothermic reactions absorb heat. Combustion reactions of hydrocarbons like methane and propane are exothermic, producing carbon dioxide, water vapor, and large amounts of heat. The heat of reaction, ΔH, indicates whether a reaction is exothermic (negative ΔH) or endothermic (positive ΔH). Bond energies represent the energy required to break bonds, while heat of combustion measures the heat released from complete combustion. A bomb calorimeter is used to accurately measure heats of combustion by igniting samples in excess oxygen. Hess's law states that the heat change of a reaction depends only onChemical energetic



Chemical energeticjslayer
╠²
Exothermic and endothermic reaction, energy level diagram, bond energy and calculatution of enthalpy change of reaction.Gases



GasesLumen Learning
╠²
This document provides an overview of key concepts related to gases, including:
- The properties of ideal gases and units of pressure like pascals and atmospheres.
- Gas laws including Boyle's, Charles', Gay-Lussac's, Avogadro's, and the ideal gas law.
- Conditions of STP and using gas laws to perform stoichiometric calculations.
- The kinetic molecular theory which explains gas properties in terms of particle motion.
- How real gases differ from ideal gases due to intermolecular forces.Different types of chemical reactions(ppt)



Different types of chemical reactions(ppt)utkarshs92
╠²
Utkarsh Singh presented on the different types of chemical reactions. There are several types including combination reactions, decomposition reactions, displacement reactions, and double displacement reactions. Combination reactions involve elements or compounds combining to form a new substance. Decomposition reactions involve breaking a substance down into simpler substances. Displacement reactions involve one element replacing another in a compound. Double displacement reactions involve ion exchange between two ionic compounds. Oxidation-reduction reactions involve the transfer of electrons between reactants. Exothermic reactions release heat while endothermic reactions absorb heat from their surroundings.Balancing Equations



Balancing EquationsBruce Coulter
╠²
A chemical equation represents a chemical reaction using formulas to show the reactants and products. It uses coefficients to indicate the ratio in which substances react or are produced, and parentheses to show the state of each substance. A chemical reaction conserves mass according to the law of conservation of mass. There are four main types of chemical reactions: synthesis, decomposition, single replacement, and double replacement.Tang 01 reversible reactions 2



Tang 01 reversible reactions 2mrtangextrahelp
╠²
The document discusses reversible reactions and chemical equilibrium. It provides examples of reversible reactions and how changing temperature affects the rates of the forward and reverse reactions. Equilibrium is reached when the rates of the forward and reverse reactions are equal. Factors like temperature, concentration, and entropy can affect whether a reaction proceeds to completion or reaches an equilibrium state.2.1 Chemical Formulas and Equations



2.1 Chemical Formulas and EquationsMelinda MacDonald
╠²
This document discusses chemical reactions and equations. It explains that chemical reactions involve substances changing partners to form new substances, while conserving mass. Chemical equations are used to represent these reactions, showing the reactants, products, and phase changes. The document also discusses how energy is often either released or absorbed during chemical reactions, depending on whether the bonds in the products are more or less stable than the reactants. Key terms like endothermic and exothermic are introduced to describe reactions that absorb or release thermal energy.Rate Of Reactions



Rate Of ReactionsEmersius
╠²
The document discusses factors that affect the rate of chemical reactions, including:
1) Temperature, concentration, pressure, particle size, and presence of catalysts can increase the rate of reaction by increasing the probability of effective collisions between reacting particles.
2) The rate of reaction generally increases with increasing temperature, concentration, and pressure or decreasing particle size because these factors increase the likelihood of collisions possessing sufficient energy to produce products.
3) Catalysts increase the rate of reaction by lowering the activation energy of the reactants through alternative reaction pathways.Chapter 8 redox reactions ppt for class 11 CBSE



Chapter 8 redox reactions ppt for class 11 CBSEritik
╠²
This document discusses oxidation-reduction (redox) reactions and oxidation states. It defines oxidation as the loss of electrons and reduction as the gain of electrons. Redox reactions involve the transfer of electrons from one atom to another. Oxidation numbers are used to track electron transfers and determine if a substance is being oxidized or reduced in a reaction. Common oxidation states of elements are discussed. Rules are provided for determining oxidation numbers based on electronegativity differences in molecules and ions.05c reversible reactions



05c reversible reactionsDr Ahmad Fahmi
╠²
This document discusses reversible chemical reactions and chemical equilibrium. It defines key terms like activation energy, exothermic and endothermic reactions, and how factors like temperature, concentration, and catalysts affect the rate and direction of reversible reactions. Specifically, it explains that at chemical equilibrium, the rates of the forward and reverse reactions are equal and application of Le Chatelier's principle describes how the system responds to changes to relieve stress.Electrolysis



Electrolysisjohnpeter208
╠²
Electrolysis is an electrochemical process where an electric current is passed through an ionic substance like molten salt or electrolyte solution, causing a non-spontaneous chemical reaction to occur. During electrolysis, ions migrate to the electrodes where they undergo oxidation or reduction reactions. Cations migrate to the cathode and gain electrons through reduction. Anions migrate to the anode and lose electrons through oxidation. The document defines key terms related to electrolysis like electrodes, electrolyte, ions, and provides examples of the half-reactions and overall reaction that occur during the electrolysis of molten sodium chloride.reaction rates



reaction ratesClaudineBagajo
╠²
The document discusses factors that affect the rate of chemical reactions, including temperature, concentration, pressure, and surface area of reactants. Increased temperature leads to more frequent and more energetic collisions between particles, speeding up reactions. Higher concentration and pressure also increase the frequency of collisions. Greater surface area of solid reactants provides more sites for collisions and reactions to occur. Catalysts increase reaction rates by reducing the activation energy required without being used up in the reaction.Chemical Reactions



Chemical ReactionsCurrituck County High School
╠²
1. The document discusses chemical reactions, including what makes something a chemical reaction, different ways to describe reactions using word equations, chemical equations, and balanced equations.
2. It describes the main types of chemical reactions - synthesis, decomposition, single replacement, double replacement, and combustion reactions - and provides examples of each.
3. Guidelines are given for writing balanced chemical equations, including identifying the reaction type and using coefficients to balance the mass on each side.Bond energies, 10 (4) 



Bond energies, 10 (4) K. Shahzad Baig
╠²
Energy required to beak a chemical bond, almost same amount of energy is used to form the same bond between reactants. Bond energies can be used to predict exothermic and endothermic nature of chemical reactions Rate of reactions



Rate of reactionsMedical Students
╠²
The document discusses the rate of chemical reactions and factors that affect it. It defines the rate of reaction as the speed at which a chemical reaction occurs. Some reactions are very fast, like wood combustion or nuclear explosions, while others are slow, such as iron rusting. The rate depends on factors like temperature, concentration of reactants, pressure, and surface area. Increasing temperature, concentration, or pressure speeds reactions up by increasing collisions between particles. Thinner solids react faster due to their larger surface area. Catalysts also increase reaction rates without being used up in the reaction. They are important in industry and biology.2.6.1 oxidation numbers



2.6.1 oxidation numbersMartin Brown
╠²
This document discusses oxidation and reduction reactions and provides rules for determining oxidation numbers:
1) Oxidation involves loss of electrons and increases oxidation number, while reduction involves gain of electrons and decreases oxidation number.
2) The sum of oxidation numbers in a molecule or ion must equal the overall charge.
3) Transition metals can have variable oxidation numbers depending on their compounds. Their oxidation states are included in names.
4) Some anomalies may occur where oxidation numbers are fractional or elements appear to gain/lose no electrons.iGCSE Chemistry Section 4 Chemical Equilibrium.ppt



iGCSE Chemistry Section 4 Chemical Equilibrium.pptHarshwardhanPhatak1
╠²
This document provides an overview of Section 4 Lesson 4 of the iGCSE Chemistry course, which covers the topic of equilibria. It begins by listing the key concepts students should understand, such as reversible reactions and how changing temperature and pressure affects equilibrium position. It then provides examples of reversible reactions, including the dehydration of hydrated copper sulfate and thermal decomposition of ammonium chloride. The majority of the document consists of explanatory text and diagrams about reversible reactions in closed systems and how increasing/decreasing temperature or pressure impacts the yield of products for endothermic/exothermic reactions. It concludes with an overview of the key topics covered in the lesson.Thermodynamics



ThermodynamicsShri Shankaracharya College, Bhilai,Junwani
╠²
hermodynamics is the branch of physics that has to do with heat and temperature and their relation to energy and work. The behavior of these quantities is governed by the four laws of thermodynamics, irrespective of the composition or specific properties of the material or system in question. The laws of thermodynamics are explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering.Bonds and energy



Bonds and energySyed Shah
╠²
Chemical reactions involve the breaking and forming of bonds. Breaking bonds requires energy and is endothermic, while forming bonds releases energy and is exothermic. Reactions can be categorized as either exothermic or endothermic depending on whether heat is released or absorbed during the reaction. Energy level diagrams are used to represent the energy changes that occur in reactions by comparing the energy of reactants to products.Electrolysis



Electrolysisshanoofsharafsrk
╠²
Electrolysis is a process where an electric current is passed through an electrolyte solution or molten salt, causing ions to migrate to the electrodes and undergo chemical reactions. During electrolysis, ions react at the electrodes by either gaining or losing electrons. Michael Faraday discovered that the mass of substance produced at the electrodes is directly proportional to the quantity of electricity passed through the electrolyte. His two laws of electrolysis state that the mass of substance liberated is directly proportional to the current over time, and that for the same quantity of electricity, the masses produced are proportional to the substances' equivalent masses. Electrolysis has many applications including metal extraction and coating materials with thin metal layers.Types of chemical reactions



Types of chemical reactionsnstahly
╠²
Introduces the 5 types of chemical reactions: synthesis, decomposition, single and double replacement, and combustion.I gcse chemistry section 5 lesson 3



I gcse chemistry section 5 lesson 3dean dundas
╠²
This document discusses synthetic polymers and their production. It begins by introducing addition polymerization, where monomers like ethene join together to form polymers like polyethene. Condensation polymerization is also discussed, where monomers join together while releasing a small molecule, using nylon as an example. Common synthetic polymers are then outlined, including their properties and uses. Polythene, polyvinyl chloride, polystyrene, and polypropylene are discussed. The document concludes by recapping the key topics of monomers, addition polymerization, and condensation polymerization.Chapter 15 Lecture- Chemical Equilibrium



Chapter 15 Lecture- Chemical EquilibriumMary Beth Smith
╠²
1. The document discusses chemical equilibrium, including the concepts of equilibrium, depicting equilibrium reactions with equations, the equilibrium constant K, and how the value of K relates to whether a reaction favors reactants or products.
2. It also covers heterogeneous equilibria involving solids or liquids, how the concentrations of solids and liquids do not appear in equilibrium expressions, and examples of heterogeneous equilibrium reactions like the decomposition of calcium carbonate.
3. The key aspects covered are the definition of chemical equilibrium as when forward and reverse reactions proceed at the same rate, the use of concentration ratios and partial pressures to define equilibrium constants Kc and Kp, and how heterogeneous reactions involve gases in equilibrium with solids or liquids.Classification of Reactions



Classification of ReactionsZB Chemistry
╠²
The document classifies different types of chemical reactions:
1) Synthesis reactions involve combining two or more reactants to form one product.
2) Decomposition reactions involve breaking down one reactant into two or more products.
3) Single displacement reactions involve one compound exchanging ions with another.
4) Double displacement reactions involve two compounds exchanging ions to form two new compounds.Types of chemical reactions document



Types of chemical reactions documentJudy Fitzpatrick
╠²
1. This document discusses different types of chemical reactions including synthesis, decomposition, single replacement, double replacement, and combustion reactions.
2. It provides examples of equations for each type of reaction and asks questions to test understanding of classifying reactions and identifying the products.
3. Key points covered include the definitions of synthesis as two reactants forming one product, decomposition as one reactant breaking into two or more products, and double replacement as two compounds exchanging ions to form two new compounds.More Related Content
What's hot (20)
Different types of chemical reactions(ppt)



Different types of chemical reactions(ppt)utkarshs92
╠²
Utkarsh Singh presented on the different types of chemical reactions. There are several types including combination reactions, decomposition reactions, displacement reactions, and double displacement reactions. Combination reactions involve elements or compounds combining to form a new substance. Decomposition reactions involve breaking a substance down into simpler substances. Displacement reactions involve one element replacing another in a compound. Double displacement reactions involve ion exchange between two ionic compounds. Oxidation-reduction reactions involve the transfer of electrons between reactants. Exothermic reactions release heat while endothermic reactions absorb heat from their surroundings.Balancing Equations



Balancing EquationsBruce Coulter
╠²
A chemical equation represents a chemical reaction using formulas to show the reactants and products. It uses coefficients to indicate the ratio in which substances react or are produced, and parentheses to show the state of each substance. A chemical reaction conserves mass according to the law of conservation of mass. There are four main types of chemical reactions: synthesis, decomposition, single replacement, and double replacement.Tang 01 reversible reactions 2



Tang 01 reversible reactions 2mrtangextrahelp
╠²
The document discusses reversible reactions and chemical equilibrium. It provides examples of reversible reactions and how changing temperature affects the rates of the forward and reverse reactions. Equilibrium is reached when the rates of the forward and reverse reactions are equal. Factors like temperature, concentration, and entropy can affect whether a reaction proceeds to completion or reaches an equilibrium state.2.1 Chemical Formulas and Equations



2.1 Chemical Formulas and EquationsMelinda MacDonald
╠²
This document discusses chemical reactions and equations. It explains that chemical reactions involve substances changing partners to form new substances, while conserving mass. Chemical equations are used to represent these reactions, showing the reactants, products, and phase changes. The document also discusses how energy is often either released or absorbed during chemical reactions, depending on whether the bonds in the products are more or less stable than the reactants. Key terms like endothermic and exothermic are introduced to describe reactions that absorb or release thermal energy.Rate Of Reactions



Rate Of ReactionsEmersius
╠²
The document discusses factors that affect the rate of chemical reactions, including:
1) Temperature, concentration, pressure, particle size, and presence of catalysts can increase the rate of reaction by increasing the probability of effective collisions between reacting particles.
2) The rate of reaction generally increases with increasing temperature, concentration, and pressure or decreasing particle size because these factors increase the likelihood of collisions possessing sufficient energy to produce products.
3) Catalysts increase the rate of reaction by lowering the activation energy of the reactants through alternative reaction pathways.Chapter 8 redox reactions ppt for class 11 CBSE



Chapter 8 redox reactions ppt for class 11 CBSEritik
╠²
This document discusses oxidation-reduction (redox) reactions and oxidation states. It defines oxidation as the loss of electrons and reduction as the gain of electrons. Redox reactions involve the transfer of electrons from one atom to another. Oxidation numbers are used to track electron transfers and determine if a substance is being oxidized or reduced in a reaction. Common oxidation states of elements are discussed. Rules are provided for determining oxidation numbers based on electronegativity differences in molecules and ions.05c reversible reactions



05c reversible reactionsDr Ahmad Fahmi
╠²
This document discusses reversible chemical reactions and chemical equilibrium. It defines key terms like activation energy, exothermic and endothermic reactions, and how factors like temperature, concentration, and catalysts affect the rate and direction of reversible reactions. Specifically, it explains that at chemical equilibrium, the rates of the forward and reverse reactions are equal and application of Le Chatelier's principle describes how the system responds to changes to relieve stress.Electrolysis



Electrolysisjohnpeter208
╠²
Electrolysis is an electrochemical process where an electric current is passed through an ionic substance like molten salt or electrolyte solution, causing a non-spontaneous chemical reaction to occur. During electrolysis, ions migrate to the electrodes where they undergo oxidation or reduction reactions. Cations migrate to the cathode and gain electrons through reduction. Anions migrate to the anode and lose electrons through oxidation. The document defines key terms related to electrolysis like electrodes, electrolyte, ions, and provides examples of the half-reactions and overall reaction that occur during the electrolysis of molten sodium chloride.reaction rates



reaction ratesClaudineBagajo
╠²
The document discusses factors that affect the rate of chemical reactions, including temperature, concentration, pressure, and surface area of reactants. Increased temperature leads to more frequent and more energetic collisions between particles, speeding up reactions. Higher concentration and pressure also increase the frequency of collisions. Greater surface area of solid reactants provides more sites for collisions and reactions to occur. Catalysts increase reaction rates by reducing the activation energy required without being used up in the reaction.Chemical Reactions



Chemical ReactionsCurrituck County High School
╠²
1. The document discusses chemical reactions, including what makes something a chemical reaction, different ways to describe reactions using word equations, chemical equations, and balanced equations.
2. It describes the main types of chemical reactions - synthesis, decomposition, single replacement, double replacement, and combustion reactions - and provides examples of each.
3. Guidelines are given for writing balanced chemical equations, including identifying the reaction type and using coefficients to balance the mass on each side.Bond energies, 10 (4) 



Bond energies, 10 (4) K. Shahzad Baig
╠²
Energy required to beak a chemical bond, almost same amount of energy is used to form the same bond between reactants. Bond energies can be used to predict exothermic and endothermic nature of chemical reactions Rate of reactions



Rate of reactionsMedical Students
╠²
The document discusses the rate of chemical reactions and factors that affect it. It defines the rate of reaction as the speed at which a chemical reaction occurs. Some reactions are very fast, like wood combustion or nuclear explosions, while others are slow, such as iron rusting. The rate depends on factors like temperature, concentration of reactants, pressure, and surface area. Increasing temperature, concentration, or pressure speeds reactions up by increasing collisions between particles. Thinner solids react faster due to their larger surface area. Catalysts also increase reaction rates without being used up in the reaction. They are important in industry and biology.2.6.1 oxidation numbers



2.6.1 oxidation numbersMartin Brown
╠²
This document discusses oxidation and reduction reactions and provides rules for determining oxidation numbers:
1) Oxidation involves loss of electrons and increases oxidation number, while reduction involves gain of electrons and decreases oxidation number.
2) The sum of oxidation numbers in a molecule or ion must equal the overall charge.
3) Transition metals can have variable oxidation numbers depending on their compounds. Their oxidation states are included in names.
4) Some anomalies may occur where oxidation numbers are fractional or elements appear to gain/lose no electrons.iGCSE Chemistry Section 4 Chemical Equilibrium.ppt



iGCSE Chemistry Section 4 Chemical Equilibrium.pptHarshwardhanPhatak1
╠²
This document provides an overview of Section 4 Lesson 4 of the iGCSE Chemistry course, which covers the topic of equilibria. It begins by listing the key concepts students should understand, such as reversible reactions and how changing temperature and pressure affects equilibrium position. It then provides examples of reversible reactions, including the dehydration of hydrated copper sulfate and thermal decomposition of ammonium chloride. The majority of the document consists of explanatory text and diagrams about reversible reactions in closed systems and how increasing/decreasing temperature or pressure impacts the yield of products for endothermic/exothermic reactions. It concludes with an overview of the key topics covered in the lesson.Thermodynamics



ThermodynamicsShri Shankaracharya College, Bhilai,Junwani
╠²
hermodynamics is the branch of physics that has to do with heat and temperature and their relation to energy and work. The behavior of these quantities is governed by the four laws of thermodynamics, irrespective of the composition or specific properties of the material or system in question. The laws of thermodynamics are explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering.Bonds and energy



Bonds and energySyed Shah
╠²
Chemical reactions involve the breaking and forming of bonds. Breaking bonds requires energy and is endothermic, while forming bonds releases energy and is exothermic. Reactions can be categorized as either exothermic or endothermic depending on whether heat is released or absorbed during the reaction. Energy level diagrams are used to represent the energy changes that occur in reactions by comparing the energy of reactants to products.Electrolysis



Electrolysisshanoofsharafsrk
╠²
Electrolysis is a process where an electric current is passed through an electrolyte solution or molten salt, causing ions to migrate to the electrodes and undergo chemical reactions. During electrolysis, ions react at the electrodes by either gaining or losing electrons. Michael Faraday discovered that the mass of substance produced at the electrodes is directly proportional to the quantity of electricity passed through the electrolyte. His two laws of electrolysis state that the mass of substance liberated is directly proportional to the current over time, and that for the same quantity of electricity, the masses produced are proportional to the substances' equivalent masses. Electrolysis has many applications including metal extraction and coating materials with thin metal layers.Types of chemical reactions



Types of chemical reactionsnstahly
╠²
Introduces the 5 types of chemical reactions: synthesis, decomposition, single and double replacement, and combustion.I gcse chemistry section 5 lesson 3



I gcse chemistry section 5 lesson 3dean dundas
╠²
This document discusses synthetic polymers and their production. It begins by introducing addition polymerization, where monomers like ethene join together to form polymers like polyethene. Condensation polymerization is also discussed, where monomers join together while releasing a small molecule, using nylon as an example. Common synthetic polymers are then outlined, including their properties and uses. Polythene, polyvinyl chloride, polystyrene, and polypropylene are discussed. The document concludes by recapping the key topics of monomers, addition polymerization, and condensation polymerization.Chapter 15 Lecture- Chemical Equilibrium



Chapter 15 Lecture- Chemical EquilibriumMary Beth Smith
╠²
1. The document discusses chemical equilibrium, including the concepts of equilibrium, depicting equilibrium reactions with equations, the equilibrium constant K, and how the value of K relates to whether a reaction favors reactants or products.
2. It also covers heterogeneous equilibria involving solids or liquids, how the concentrations of solids and liquids do not appear in equilibrium expressions, and examples of heterogeneous equilibrium reactions like the decomposition of calcium carbonate.
3. The key aspects covered are the definition of chemical equilibrium as when forward and reverse reactions proceed at the same rate, the use of concentration ratios and partial pressures to define equilibrium constants Kc and Kp, and how heterogeneous reactions involve gases in equilibrium with solids or liquids.Viewers also liked (13)
Classification of Reactions



Classification of ReactionsZB Chemistry
╠²
The document classifies different types of chemical reactions:
1) Synthesis reactions involve combining two or more reactants to form one product.
2) Decomposition reactions involve breaking down one reactant into two or more products.
3) Single displacement reactions involve one compound exchanging ions with another.
4) Double displacement reactions involve two compounds exchanging ions to form two new compounds.Types of chemical reactions document



Types of chemical reactions documentJudy Fitzpatrick
╠²
1. This document discusses different types of chemical reactions including synthesis, decomposition, single replacement, double replacement, and combustion reactions.
2. It provides examples of equations for each type of reaction and asks questions to test understanding of classifying reactions and identifying the products.
3. Key points covered include the definitions of synthesis as two reactants forming one product, decomposition as one reactant breaking into two or more products, and double replacement as two compounds exchanging ions to form two new compounds.Chemical Reactions 1196945876279030 3



Chemical Reactions 1196945876279030 3Laura Verastegui
╠²
This document discusses different types of chemical reactions including synthesis, decomposition, single displacement, and double displacement reactions. It provides examples of each type of reaction using chemical equations. It also describes the activity series of metals and how reactivity determines which metals can displace other metals in single displacement reactions.CHEMICAL EQUATIONS AND REACTIONS



CHEMICAL EQUATIONS AND REACTIONSAditee Chakurkar
╠²
1. Chemical reactions involve chemical changes that result in the formation of new substances.
2. Chemical equations are used to represent chemical reactions, with reactants on the left side of the arrow and products on the right. These equations must be balanced and follow the law of conservation of mass.
3. There are several types of chemical reactions including combination, decomposition, displacement, and oxidation-reduction. Combination reactions involve elements or compounds reacting to form a single product, while decomposition reactions involve a single reactant breaking down into simpler products.Solutions



SolutionsCurrituck County High School
╠²
This document defines key concepts related to solutions, including:
- A solution is a homogeneous mixture of a solvent and one or more solutes. Common solvents are water.
- The process of dissolving a solute involves the solute and solvent particles separating and the solvent surrounding and solvating the solute particles. This process can be endothermic or exothermic.
- Solubility depends on properties of the solute and solvent as well as temperature and pressure. Solubility curves show how solubility changes with temperature. Saturation occurs when a solution contains the maximum amount of solute possible.Classification of reactions and reactors



Classification of reactions and reactorsengrktk
╠²
The document discusses challenges in chemical reaction engineering and the potential of membrane reactors. It notes that while chemical reaction engineering is mature, reaction rates are often too low and conversions are thermodynamically limited. Membrane reactors could help address issues by retaining homogeneous catalysts, separating products to shift equilibrium, and integrating reaction and separation steps. The document also provides classifications of chemical reactions and conventional reactor types. It focuses on how membrane reactors may improve reaction selectivity and energy efficiency over traditional approaches.OFFSHC gets briefed on IEDs



OFFSHC gets briefed on IEDsOFFSHC
╠²
On September 11, Corporal Robert Tye, Oklahoma County SheriffŌĆÖs Office, provided the OFFSHC a presentation about improvised explosive devices (IED). He discussed the components of and how to recognize an IED. Corporal Tye also displayed examples of inert IEDs that the Oklahoma County Sheriff may have recognized in Oklahoma.Powerpoint chemicals of-life



Powerpoint chemicals of-lifeMagdalena Ravagnan
╠²
The document discusses the key chemicals found in living cells - carbohydrates, proteins, lipids, water and enzymes. It provides details on their structures and functions. Carbohydrates include sugars like glucose and serve as an energy source. Proteins are made of amino acids and are essential components of cells. Lipids contain fatty acids and glycerol. Enzymes are protein catalysts that allow chemical reactions to occur faster in cells. They function by temporarily binding to substrates to facilitate the formation or breakdown of molecules.Displacement reaction



Displacement reactionsuryacad
╠²
1) Displacement reactions can be classified as metal-metal displacement reactions or metal-nonmetal displacement reactions.
2) In metal-metal displacement reactions, more reactive metals displace less reactive metals according to the reactivity series. For example, copper displaces iron when an iron bar is placed in copper sulfate solution.
3) In metal-nonmetal displacement reactions, metals can displace hydrogen from water, with more reactive metals like sodium displacing hydrogen even at room temperature, while less reactive metals like iron only displacing hydrogen when heated.Chemical Reaction Basics



Chemical Reaction BasicsBen Wildeboer
╠²
An attempt to make the description of chemical reactions a mini-story. Not the best story by any means, but it was pretty well received by the students.Solution & Solubility



Solution & Solubilityitutor
╠²
A solution is a homogeneous mixture of two or more substances, where the solute is dispersed uniformly throughout the solvent. The solubility of a solute is dependent on temperature, pressure, and the nature of the solute and solvent. Solubility is expressed as the maximum grams of solute that will dissolve per 100 grams of solvent. Colligative properties, such as boiling point elevation and freezing point depression, depend only on the number of solute particles and not their identity.Organic reactions and mechanisms



Organic reactions and mechanismsKandarp Vyas
╠²
The document discusses organic reactions and reaction mechanisms. It defines nucleophiles and electrophiles, and provides examples of each. It then summarizes several common types of organic reactions including addition reactions, substitution reactions, elimination reactions, and aromatic substitutions. The mechanisms and examples of nucleophilic addition, electrophilic addition, nucleophilic substitution, and electrophilic aromatic substitutions like nitration, sulfonation, and halogenation are described in detail.IB Chemistry on Standard Reduction Potential, Standard Hydrogen Electrode and...



IB Chemistry on Standard Reduction Potential, Standard Hydrogen Electrode and...Lawrence kok
╠²
The document discusses standard electrode potentials and how they are measured. It explains that the standard hydrogen electrode is used as a reference with a potential of 0 V. Other half-cell potentials are measured against this to determine their standard electrode potential. Common half-cells include metal/metal ion, gas/ion, and ion/ion systems. Standard conditions of 1 M concentrations, 1 atm pressure, and 298K temperature must be used. The potentials of zinc/zinc ion, iron III/iron II, and chlorine/chloride ion half-cells are given as examples.Similar to Classification of Chemical Reactions (20)
Types of Reactions PPT.pdf_┘Ī┘Ī┘ó┘ó┘Ā┘Ā┘Ā┘ż.pptx



Types of Reactions PPT.pdf_┘Ī┘Ī┘ó┘ó┘Ā┘Ā┘Ā┘ż.pptxsalehalgabri02
╠²
There are five main types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion. A chemical reaction occurs when atoms bond together to form new compounds or when compounds separate to form other compounds. Chemical reactions can be identified by certain characteristics like gas produced, light produced, temperature change, color change, or precipitate formed.Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactions



Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactionsMoulyaT
╠²
A chemical reaction involves one or more reactants forming new products with different properties. Chemical equations represent reactions symbolically, with balanced equations showing equal numbers of atoms on both sides. There are several types of reactions including combination, decomposition, exothermic/endothermic, displacement, neutralization, and oxidation-reduction. Identification of a reaction can occur through changes in state, color, gas evolution, temperature change, or precipitate formation.Tejendra BIOCHEM.pptx



Tejendra BIOCHEM.pptxTejendra4
╠²
Bio chemistry ppt
Oxidation ppt
Reduction ppt
Hydrolysis ppt
Science ppt
Class 11 Chemistry ppt
Redox reaactions ppt
Chemistry ppt
oxidizing reagents ppt
Reducing reagents ppt
Oxidation ppt
Reduction ppt
Oxidation number ppt
presentation on chemical reactions and equations by - Aryan dhiman.pdf



presentation on chemical reactions and equations by - Aryan dhiman.pdfsanjeevkumar403105
╠²
Chemical reactions and equations Chemical equations and reactions 



Chemical equations and reactions GIRISH
╠²
1. Chemical reactions involve chemical changes where reactants are converted into products. Signs of a chemical reaction include changes in color, gas evolution, temperature changes, and state changes.
2. A word equation represents the reactants and products of a chemical reaction using chemical names. A balanced chemical equation uses formulas to represent the reactants on the left and products on the right, separated by an arrow.
3. There are several types of chemical reactions including combination, decomposition, displacement, and redox reactions. Combination reactions involve reactants combining to form compounds while decomposition reactions involve splitting compounds into simpler substances.chemical equation and reaction



chemical equation and reactioncooliohan
╠²
Chemical reactions involve the rearrangement of atoms and the formation of new substances. There are several types of chemical reactions including combination, decomposition, single displacement, double displacement, and redox reactions. Balanced chemical equations are used to represent these reactions and must satisfy the law of conservation of mass.Chemical reactions and equations



Chemical reactions and equationsAyushmanTiwari4
╠²
Hello friends this is the notes of the chapter chemical reactions and equations .
I hope this will help you in your examinations
Stay safe, Stay home, Stay connected Mirakle puhan sc.



Mirakle puhan sc.Mirakle Puhan
╠²
1. Chemical reactions can be represented through word equations, chemical equations, and balanced chemical equations.
2. There are several types of chemical reactions including combination, decomposition, displacement, and oxidation-reduction.
3. Word equations show the reactants and products of a chemical reaction using chemical names. Chemical equations use chemical formulas and are balanced to satisfy the law of conservation of mass.Chemical Reactions Notes



Chemical Reactions Notesduncanpatti
╠²
- Chemical reactions involve reactants transforming into products through rearrangement of atoms.
- There are five main types of chemical reactions: combination, decomposition, single replacement, double replacement, and combustion.
- The type of reaction can be identified based on the reactants. Balancing chemical equations ensures the same number and type of atoms are on both sides.Chapter 11



Chapter 11SHERIFA s
╠²
This document describes chemical reactions and equations. It discusses:
1. The components of chemical equations including reactants and products.
2. The different types of chemical equations including word equations, skeleton equations, and chemical equations.
3. How to balance chemical equations by ensuring equal numbers of each type of atom on both sides of the reaction arrow.
4. The five major types of chemical reactions - combination, decomposition, single replacement, double replacement, and combustion.
5. How to predict whether a precipitation reaction will occur based on solubility rules for ionic compounds.introduction to puckers.pptx



introduction to puckers.pptxitzkuu01
╠²
1) The document discusses various types of evidence that indicate a chemical reaction has occurred, including temperature change, gas formation, color change, and precipitate formation.
2) It describes word equations, skeleton equations, and how to translate between the two. Balancing equations requires using coefficients to satisfy the law of conservation of mass.
3) Key characteristics of the main types of chemical reactions - synthesis, decomposition, single replacement, double replacement, and combustion - are outlined.Chemical reaction and equation.pdf class 10



Chemical reaction and equation.pdf class 10ta8789552862
╠²
This is for student of class 10 for division in very short time . This is the notes of chemical reaction and equation which is chapter number first of chemistry of class 10 fully based on NCERT. This help students to clear the concepts and take examples of the reactions and other. From this PDF students can easily score up to 80 to 90%. This PDF is made by a student of class 10 so they can easily understand the problem of other student. Thank youChemical Reaction



Chemical ReactionBhandari Gunjan
╠²
This presentation is prepare by Gunjan Bhandary studying in class 9 of Baylor International Academy located in Banepa, NepalClassification of Chemical Reactions
- 1. WhatŌĆÖs in a name?The FIVE General Types of Chemical Reactions
- 2. Definition: A reaction in which TWO or more substances combine to form a SINGLEnew compound.Characteristics:General Formula: A + X -> AXSynthesis Reaction
- 3. Example of Synthesis ReactionFormula Equation: A chemical equation written in proper chemical formulas ONLY. Na + Cl2 -> NaClNotice how the product, NaCl: is chemically different from both the reactants, Sodium - METAL, and Chlorine ŌĆō GREEN GAS.
- 4. Additional Example of Synthesis ReactionsExample #2: Fe + O 2 -> Fe3O2This is the chemical process we call, RUST.
- 5. H2O -> H2 +O2 This reaction only takes place during electrolysis; when an electrical current is used. Non-Example of Synthesis Reaction** The Electrolysis of Water - Movie
- 6. Definition: A reaction in which ONE substance breaks down to form TWO or more simpler substances. Characteristics:General Formula: AX -> A + XUsually occurs in the presence of heat or electricity.Decomposition Reaction
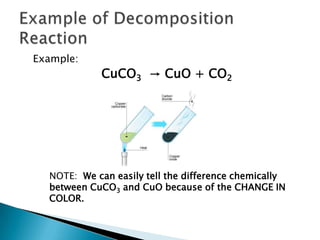
- 7. Example of Decomposition ReactionExample:CuCO3 -> CuO + CO2NOTE: We can easily tell the difference chemically between CuCO3 and CuO because of the CHANGE IN COLOR.
- 8. Additional Example of Decomposition ReactionsFormula Equation:I4O9-> I2O6 + I2
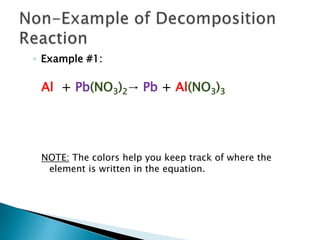
- 9. Example #1: Al + Pb(NO3)2-> Pb + Al(NO3)3NOTE: The colors help you keep track of where the element is written in the equation. Non-Example of Decomposition Reaction
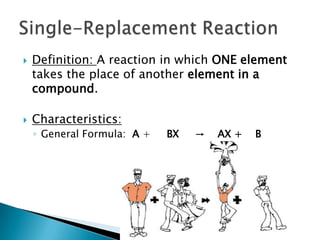
- 10. Definition: A reaction in which ONE element takes the place of another element in a compound. Characteristics:General Formula: A + BX -> AX + BSingle-Replacement Reaction
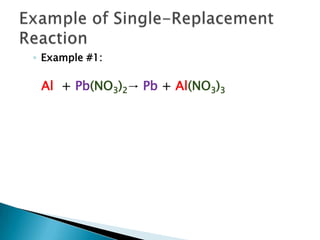
- 11. Example of Single-Replacement ReactionExample #1: Al + Pb(NO3)2-> Pb + Al(NO3)3
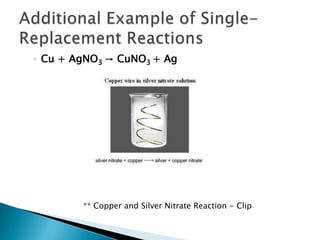
- 12. Additional Example of Single-Replacement ReactionsCu + AgNO3 -> CuNO3 + Ag** Copper and Silver Nitrate Reaction - Clip
- 13. Non-Example of Single-Replacement ReactionPb(NO3)2+ KI -> PbI2+ KNO3
- 14. Definition: A reaction in which appears exchange of atoms or ions between two compounds has occurred. Characteristics:General Formula: AY + BX -> AX + BYDouble-Replacement Reaction
- 15. Example of Double -Replacement ReactionPb(NO3)2+ KI -> PbI2+ KNO3
- 16. Additional Example of Double-Replacement ReactionsFormula Equation: NaCl+ AgF-> NaF+ AgCl
- 17. Formula Equation: Non-Example of Double-Replacement Reaction1) CuCO3 -> CuO + CO22) Na + Cl2 -> NaCl
- 18. Definition: A combustion reaction is when oxygen combines with another compound to form water and carbon dioxide.* Characteristics:A common combustion reactant is oxygen.Two common combustion products are carbon dioxide and water.Combustion Reaction
- 19. Additional Examples of Combustion ReactionExample #1: CH4 + O2 -> CO2+ H2OExample #2: C3H8+ O2 -> CO2 + H2O
- 20. Non-Example of Combustion Reaction1) CuCO3 -> CuO+ CO22) Na + Cl -> NaCl3) NaCl+ AgF-> NaF+ AgCl
- 21. In-Class AssignmentComplete Questions 1-10 on Worksheet: Classification of Chemical ReactionsSample Problem - Q10 Ca(OH)2 + H2SO4-> CaSO4 + H2O





