Clinical Evaluation Report (CER) - Decode the Step-wise Approach for Compliance
- 1. Clinical Evaluation Report (CER)Decode the Step-wise Approach for Compliance Date 11th December 2018
- 2. Regulatory : CONSULTING | SUBMISSIONS | AFFAIRS | SUPPORT | INTELLIGENCE | LABELING | SOFTWARE 2www.freyrsolutions.com | sales@freyrsolutions.com | CER- Industry Reception in last 2 years 04 Clinical Data, Evaluation and Evidence - Overview 05 Agenda PMS Data – Key Input for CER 06 Clinical Evaluation - - Key component for Clinical EvidenceE 07 Key High Lights of MEDDEV 2.7/1 REV 4 08 Clinical Evaluation Report- Key Characteristics 09 Focus points for a “compliant CER” 12 Stage wise approach for CER Authoring 13 Key Impact Elements for CER Projects 14 Requirement of Enablers in CER Projects 20 Next steps 20 Current Challenges 14
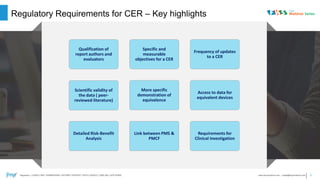
- 3. Regulatory : CONSULTING | SUBMISSIONS | AFFAIRS | SUPPORT | INTELLIGENCE | LABELING | SOFTWARE 3www.freyrsolutions.com | sales@freyrsolutions.com | Regulatory Requirements for CER – Key highlights Qualification of report authors and evaluators Specific and measurable objectives for a CER Frequency of updates to a CER Scientific validity of the data ( peer- reviewed literature) More specific demonstration of equivalence Access to data for equivalent devices Detailed Risk-Benefit Analysis Link between PMS & PMCF Requirements for Clinical Investigation
- 4. Regulatory : CONSULTING | SUBMISSIONS | AFFAIRS | SUPPORT | INTELLIGENCE | LABELING | SOFTWARE 4www.freyrsolutions.com | sales@freyrsolutions.com | Clinical Evaluation Report – Key characteristics Clinical evaluation is the assessment and analysis of clinical data needed to verify the clinical safety and performance of your medical device. Live Document Stand Alone Document Require regular reviews and updates through out the device life cycle Require updates from post-market surveillance activities
- 5. Regulatory : CONSULTING | SUBMISSIONS | AFFAIRS | SUPPORT | INTELLIGENCE | LABELING | SOFTWARE 5www.freyrsolutions.com | sales@freyrsolutions.com | Questions Would you like to decode the step-wise approach for CER? We Appreciate Your Interest to Know More on ”Clinical Evaluation Report (CER)”. To Access the Entire Session, Click Here. https://www.freyrsolutions.com/webinars/clinical- evaluation-report-cer-decode-the-step-wise- approach-for-compliance
- 6. THANK YOU sales@freyrsolutions.com +1 908 483 7958 www.freyrsolutions.com Freyr Locations USA Canada UK Germany UAE Malaysia South Africa Singapore Slovenia +1 908 483 7958 +1 778 308 4671 +44 2037 0123 79 +49 618 170 79007 +1 908 483 7958 +603 9212 5527 Mexico +52 554 161 3365 +27 105 002 556 +65 315 894 72 +386 360 004 05 Austria +1 908 483 7958 Sri Lanka +1 908 483 7958 India +91 40 4848 0999