Congenital nevus Melonocytic nevus Birth marks
- 2. INTRODUCTION ŌĆó Congenital or acquired melanocytic neoplasms. ŌĆó Common acquired moles: well-demarcated, uniformly tan-brown papules (Ōēż 6mm). ŌĆó Have several variants: ŌĆó Congenital nevus ŌĆó Blue nevus ŌĆó Spindle & Epithelioid cell nevus (Spitz nevus) ŌĆó Halo nevus ŌĆó Dysplastic nevus
- 3. PATHOGENESIS: ŌĆó Many nevi: acquired mutations in BRAF or NRAS, genes involved in RAS signaling. ŌĆó Lead to a limited period of proliferation ŌĆó --> Followed by permanent growth arrest (most cases) ŌĆó --> due to: Accumulation of p16/INK4a (inhibitor of CDKs).
- 4. MORPHOLOGY ŌĆó Arise from basal melanocytes. ŌĆó Nevus cells: rounded cells exhibiting uniform nuclei and inconspicious nuclei ŌĆó Nevi mature through characteristic stages: ŌĆó Junctional nevi: nests of nevus cells at DE junction --> earliest lesion. ŌĆó Compound nevi: develop as nests or cords of melanocytes that extend into the underlying dermis. ŌĆó Dermal nevi: epidermal component is lost.
- 6. COMPOUND NEVUS: GROSS:- RAISED & DOME SHAPED LESION. HISTO:- BOTH JUNCTIONAL AND DERMAL COMPONENTS
- 7. INTRA-DERMAL NEVUS: Gross:- Raised domed shaped. Histo:- Only dermal component.
- 8. Contd... ŌĆó As nevus cells enter the dermis:- ŌĆó Undergo maturation: ŌĆó Larger, pigmented cells --> smaller non-pigmented cells --> Spindle shaped cells --> resemble neural tissue (Neurotization). ŌĆó In comparision, Melanoma exhibit little to no maturation.
- 9. TYPE A CELLS NUCLEUS: ROUND TO OVAL ,SMALLER THAN KERATINOCYTES , FINELY DISPERSED CHROMATIN, INCONSPICUOUS NUCLEOLI. CYTOPLASM: PROMINENT AND CONTAINS COARSE GRANULES TYPE B CELLS NUCLEUS: SMALL AND ROUND. CYTOPLASM: SCANT. PRESENT IN DERMIS. TYPE C CELLS SPINDLE SHAPED. BASE OF NEVUS (DERMIS).
- 10. DYSPLASTIC NEVUS ŌĆó Larger (> 5mm) than most acquired nevi. ŌĆó Flat macule to slightly raised papules with veriegated pigmentation and irregular borders. ŌĆó Both in sun-exposed and protected skin. ŌĆó Can number in hunderds: Dysplastic nevus syndrome. ŌĆó 50% develop Melanoma by 60 years of age. ŌĆó Most lesions clinically stable and sporadic isolated lesions have low risk of malignant transformation.
- 11. PATHOGENESIS ŌĆó AD disorder often a/w mutations in proteins a/w cell cycling. ŌĆó CDKN2A --> p16/INK4, CDK4. ŌĆó Acquired NRAS & BRAF mutations also common. ŌĆó Not all germline mutation in CDKN2A / CDK4 --> Dysplastic nevi. ŌĆó Not all Dysplastic nevi --> have mutations in these genes. ŌĆó Means: other modifiers genes also involved (eg. TERT over-expression).
- 12. MORPHOLOGY ŌĆó Histologically, most are compound nevi exhibiting architectural and cytological atypia. ŌĆó Enlarged and fused nests of nevus cells. ŌĆó Lentigenous melanocytic hyprtplasia. ŌĆó Linear papillary dermal fibrosis. ŌĆó Pigmant incontinence: release of melanin from dead melanocytes in dermis.
- 13. DYSPLASTIC NEVUS: GROSS:- Flat Macules to Target like Lesions with Darker Raised Centre & Irregular Flat Periphery. HISTO:- Dermal fibrosis, Inflammation, and Proliferation of melanocytes at D-E Jnx, with Bridging of Rete Ridges.
- 14. DYSPLASTIC NEVUS: HISTO:- Dermal fibrosis, Inflammation, and Proliferation of melanocytes at D-E Jnx, with Bridging of Rete Ridges.
- 15. MELANOMA ŌĆó Malignant melanocytic tumor. ŌĆó Most common site: Skin. ŌĆó Can also occur in: Oral & anogenital mucosal surfaces, esophagus, meninges and eyes. ŌĆó Incidence of cutaneous malignant melanoma increasing.
- 16. PATHOGENESIS: DRIVER MUTATIONS AFFECTS 3 PATHWAYS Mutations that disrupt cell cycle control genes. CDKN2A GENE MUTATION IN 40% CASES OF AD MELANOMA & 10% SPORADIC CASES NET EFFECT IS INCREASED MELANOCYTE PROLIFERATION. Mutations that activate pro- growth signaling pathways. ABBERANT INCREASE IN RAS AND PI3K/AKT SIGNALLING. ACTIVATING MUTATIONS IN BRAF. ACTIVATING MUTATIONS OF NRAS. NON-SUN EXPOSED AREAS SHOW MUTATIONS UPSTREAM OF RAS IN RTK. NET EFFECT IS INCREASED MELANOCYTE PROLIFERATION & SURVIVAL. Mutations that activate telomerase. MUTATIONS IN PROMOTER TERT GENE ACTIVATES TELOMERASE IN NEARLY 70% OF TUMORS ACT AS ANTIDOTE TO SENESCENCE
- 17. Contd... ŌĆó Growth factors activate signaling circuits involving RTK, RAS, and two key downstream pathways that include BRAF and PI3K. ŌĆó Proteins indicated by asterisks are mutated in melanoma. ŌĆó Components of these pathways that are being targeted by drugs are indicated.
- 18. WARNING SIGNS OF MELANOMA
- 19. GROSS FINDINGS ŌĆó UNLIKE BENIGN NEVI THEY SHOW STRIKING VARIATIONS IN SHADES OF BLACK BROWN RED DARK BLUE AND GRAY. ŌĆó ZONES OF WHITE OR FLESH COLOURED HYPOPIGMENTATION ALSO APPEARS SOMETIMES DUE TO FOCAL REGRESSION OF TUMOR. ŌĆó BORDERS ARE IRREGULAR AND NOTCHED UNLIKE SMOOTH ROUND AND UNIFORM BORDERS OF MELANOCYTIC NEVUS
- 20. MORPHOLOGY ŌĆó Composed of: ŌĆó Large cells. ŌĆó With expanded, irregular nuclei. ŌĆó Containing peripherally clumped chromatin. ŌĆó Have prominent eosinophilic nucleoli. ŌĆó Progress from radial to vertical growth pattern.
- 21. RADIAL GROWTH ŌĆó Horizontal spread within the epidermis and superficial dermis. ŌĆó Tumor cells typically lack the capacity to metastasize. ŌĆó Lesions include: ŌĆó Lentigo maligna: indolent lesion on the face that may not progress for decades. ŌĆó Superficial spreading: mc form, usually involve sun-exposed skin. ŌĆó Acral and Mucosal lentiginious melanoma: unrelated to sun exposure.
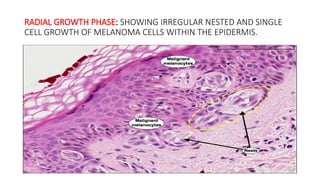
- 22. RADIAL GROWTH PHASE: SHOWING IRREGULAR NESTED AND SINGLE CELL GROWTH OF MELANOMA CELLS WITHIN THE EPIDERMIS.
- 23. LENTIGO MALIGNA ŌĆó SUN EXPOSED AREA OF ELDERLY WHITE MC IN CHEEKS. ŌĆó FLAT SLOW GROWING. ŌĆó Histo: PROLIFERATION OF ATYPICAL MELANOCYTES IN BASAL LAYER, DISTRIBUTED INDIVIDUALLY AS WELL AS IN NESTS. ŌĆó WHEN CONFINED TO EPIDERMIS: LENTIGO MALIGNA (FORM OF MIS). ŌĆó WHEN A DERMAL COMPONENT IS PRESENT: LENTIGO MALIGNA MELANOMA.
- 24. SUPERFICIAL SPREADING MELANOMA: Pagetoid appearance of melanocytes in superficially spreading malignant melanoma.
- 26. VERTICAL GROWTH ŌĆó Occurs unpredictably. ŌĆó Characterized by dermal invasion of expanding clonal mass of cells. ŌĆó Lack cellular maturation. ŌĆó Often have capacity to metastasize. ŌĆó Probability of distal spread corelates with depth of invasion (Breslow thickness).
- 28. PROGNOSTIC FACTORS ŌĆó Breslow thickness (thinner is better). ŌĆó Number of mitosis (<1/mm2 ). ŌĆó Evidence of regression (absent). ŌĆó Ulceration (absent). ŌĆó Presence of tumor-infiltrating L╬” (many). ŌĆó Gender (female). ŌĆó Location (extremity). ŌĆó Sentinal LN mets (absent).
- 29. CLINICAL FEATURES ŌĆó Warning signs: ABCDE (if rapid). ŌĆó Cutaneous melanoma: most Asx, pain, pruritis. ŌĆó Majority: >10mm at Dx, usually a/w olor variegations. ŌĆó Most consistent sign: recent change in size, shape or color. ŌĆó Borders: often irregular &/or notched, with zones of hypopigmentation (d/t focal regression)
- 32. SQUAMOUS CELL CARCINOMA (SCC)