Counter current extraction
- 1. LUCKY KUMAR M.PHARM 1ST YEAR (UIPS) PANJAB UNIVERSITY
- 2. Counter current extraction • Counter current extraction is a method of multiple liquid- liquid extraction. • Separation of components having variable solubility in two immiscible liquid phases is achieved. • In the counter current extraction two immiscible solvents flow in an opposite direction in multiple stages, (after several stages pure A and B solvents can be obtained)
- 3. • In liquid- liquid extraction the solvent is used to extract another liquid phase. • To distribution of active principles between water and organic solvent depends on the hydrophilic groups present in the constituent molecules. • If hydrophilic groups are ionisable ,PH will be an important factor. • If ionisation constants of isomers are different then separation can be achieved.
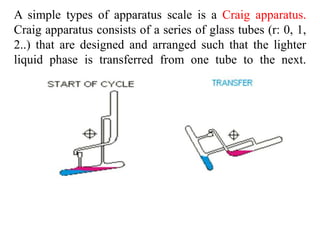
- 4. Instrumentation A method of multiple liquid-liquid extractions is countercurrent extraction, which permits the separation of substances with different distribution coefficients (ratios). A clever design known as Craig apparatus is used for this purpose (Lyman C. Craig, 1943).
- 5. A simple types of apparatus scale is a Craig apparatus. Craig apparatus consists of a series of glass tubes (r: 0, 1, 2..) that are designed and arranged such that the lighter liquid phase is transferred from one tube to the next.
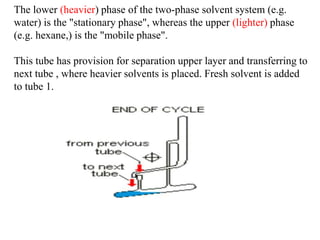
- 6. The lower (heavier) phase of the two-phase solvent system (e.g. water) is the "stationary phase", whereas the upper (lighter) phase (e.g. hexane,) is the "mobile phase". This tube has provision for separation upper layer and transferring to next tube , where heavier solvents is placed. Fresh solvent is added to tube 1.
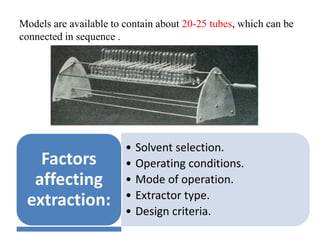
- 7. Models are available to contain about 20-25 tubes, which can be connected in sequence . • Solvent selection. • Operating conditions. • Mode of operation. • Extractor type. • Design criteria. Factors affecting extraction:
- 8. Boiling point , stability. Density,, Interfacial tension. toxicity cost viscosity, compatibility with product Other factors affecting solvent selection are : :
- 9. Disadvantages 1. Organic solvents are costly and inflammable. 2. Ideal solvent must have high partition coefficient. i.e. the solvent must have high affinity for the required products. Aqueous phases has negligible solubility. 3. Normally aqueous phase is large and the quantity of valuable products is low .
- 10. Application • Isolation of antibiotics (penicillin G) from the aqueous fermentation broth using immiscible solvent (amyl acetate or butyl acetate) • Isolation of chemical compounds from the aqueous systems using small quantities of organic solvents in the production of synthetic drugs and intermediates. • In petroleum industry products having different chemical structure but about the same boiling range are separated. • Aromatic compounds are recovered from paraffin fraction of the petroleum oil.
- 11. • Separation of components from synthetic mixtures. • Separation of components from plant extract. • Purification of compounds (removal of impurities)
- 12. THANK YOU