Cyanide toxicity in plants
- 1. Presented to: Dr. Mansoor Hameed Presented By: Ayesha Siddiqui Cyanide toxicity in plants
- 5. Organic Cyanides ï Organic cyanides are usually called nitriles; in these, the CN group is linked by a covalent bond to a carbon- containing group, such as methyl (CH3) in methyl cyanide (acetonitrile). ï In nature, substances yielding cyanide are present in certain seeds, such as the pit of the wild cherry and the seeds of apples.
- 6. Toxicity of Cyanide Ions ï Toxicity resides in the release of Cyanide ion, CN- ï Inorganic Cyanides are potentially toxic as they have capability of removal of ion when in a medium ï Organic nitriles do not readily release cyanide ions, and so have low toxicities. By contrast, compounds such as trimethylsilyl cyanide (CH3)3SiCN readily release HCN or the cyanide ion upon contact with water
- 8. Cyanogenic glycosides in edible plants
- 9. Mechanism of release of CN- ï Cyanogenic glycosides in plants are stored in the vacuole, but, if the plant is attacked, they are released and become activated by enzymes in the cytoplasm. These remove the sugar part of the molecule and release toxic hydrogen cyanide. ï Storing them in inactive forms in the vacuole prevents them from damaging the plant under normal conditions.
- 10. Occurrence of Cyanogenic glycosides in plants Family Plant name Cyanogenic glycoside Rosaceae Peach (Prunus persica) Amygdalin Plum (Prunus domestica ) Amygdalin Bitter Almond (P. amygdalus var.amara) Amygdalin Apricot (Prunus armeniaca) Amygdalin Prunus serotina Prunasin Prunus laurocerasus Prulaurasin
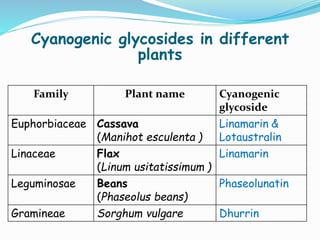
- 11. Cyanogenic glycosides in different plants Family Plant name Cyanogenic glycoside Euphorbiaceae Cassava (Manihot esculenta ) Linamarin & Lotaustralin Linaceae Flax (Linum usitatissimum ) Linamarin Leguminosae Beans (Phaseolus beans) Phaseolunatin Gramineae Sorghum vulgare Dhurrin
- 12. Natural occurrence of cyanide in Plants
- 13. substances yielding cyanide are present in seeds of apples I have Cyanide in me
- 16. Almond (Prunus amygdalus) The bitter almond emyzne eht sniatnocnislume which, in the presence of water, acts on amygdalin, yielding 2 glucose, cyanide and the essential oil of bitter almonds, which is nearly pure benzaldehyde.
- 18. ï Cyanogenic glycosides might serve as nitrogen storage compound esp. in seeds (e.g. linamarin, linustatin etc) ï Plant protection against herbivores, pathogens, and competitors
- 20. 3 Stages of Respiration 1. Glycolysis ï cytoplasm ï with or without oxygen present ï breaks glucose (6C) into 2 pyruvates (3C) 2. TCA Cycle ï mitochondrial matrix ï only if oxygen present ï converts pyruvate via acetyl CoA into CO2; generates NADH and FADH2 3. Electron Transport Chain ï mitochondrial membranes = cristae ï transfers electrons from NADH and FADH2 to reduce O2 to H2O and generate ATP
- 23. Cytochrome c oxidase ï It receives an electron from each of four cytochrome c molecules, and transfers them to one oxygen molecule, converting molecular oxygen to two molecules of water. In the process, it binds four protons from the inner aqueous phase to make water, and in addition translocates four protons across the membrane, helping to establish a transmembrane difference of proton electrochemical potential that the ATP synthase then uses to synthesize ATP.
- 26. The binding of cyanide to this enzyme prevents transport of electrons from cytochrome c to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ATP for energy.
- 28. An âalternate pathâ (aka, the cyanide resistant path) de-couples respiratory electron transport from ATP production. This pathway produces O2, but not ATP. It can serve as an âenergy overflow valveâ when supply exceeds demand â but it results in a net loss of energy from the plant. Is this a relic âerrorâ or an important physiological function? An âalternative oxidaseâ (AOX) accepts electrons coming from complex II, preventing them from getting to complex III
- 29. Importance of Cyanide Resistant Respiration ï Its plays important role in the growth of roots. ï In fungi, the ability of the alternative oxidase to bypass inhibition of parts of the electron transport chain can contribute to fungicide resistance. E.g. This is seen in the strobilurin fungicides that target complex III, such as azoxystrobin, picoxystrobin and fluoxastrobin. However, as the alternative pathway generates less ATP, these fungicides are still effective in preventing spore germination, as this is an energy-intensive process.
- 30. When it flowers, the Philodendron flower heats to as high as 46 C (115 F). The heat protects the flowers from freezing at night and disperses compound that attract polinators Light energy â> Heat Energy is captured from light by Philodendron leaves and used for life processes and growth