D09.06.05.presentation
- 1. The High-Temperature Electrolysis Program at INL: Observations on Performance Degradation and Summary of INL-Sponsored Degradation Workshop J. E. OâBrien C. M. Stoots, J. S. Herring, K. G. Condie, G. K. Housley, M. G. McKellar, M. S. Sohal, J. J. Hartvigsen RelHy Workshop on High Temperature Water Electrolysis Limiting Factors June 9 â 10, 2009
- 2. High-Temperature Electrolysis INL has been designated as the lead laboratory for High-Temperature Electrolysis (HTE) research and development, under the DOE Nuclear Hydrogen Initiative (NHI)
- 3. INL HTE Research Scope Experimental CFD Simulation Demonstration and Scale-Up System Modeling
- 4. System Modeling Process flow diagram for the helium-cooled reactor / direct Brayton / HTE system with air sweep (reference case).
- 5. System Analysis Results Overall Hydrogen Production Efficiencies, HTE Reference Case, as a function of Cell Voltage 0.54 air swp, adiabatic, ASR 0.25 overall hydrogen production efficiency (LHV) LHV ηH = air swp, adiabatic, ASR 1.25 2 FVop (1 / ηth â 1) + HHV air swp, isothermal, ASR 0.25 0.52 air swp, isothermal, ASR 1.25 no swp, adiabatic, ASR 0.25 no swp, adiabatic, ASR 1.25 no swp, isothermal, ASR 0.25 0.5 no swp, isothermal, ASR 1.25 simple thermo analysis 0.48 0.46 0.44 1 1.05 1.1 1.15 1.2 1.25 1.3 1.35 1.4 per-cell operating voltage The red line follows from the definition of the overall thermal-to-hydrogen efficiency η = LHV and direct application of the first law â Qi H i
- 6. System Analysis Results Overall Hydrogen Production Efficiencies HTE Reference Case (air sweep) vs Hydrogen Production Rate vs Steam Utilization 3 0.5 hydrogen production rate, m /hr overall hydrogen production efficiency 0 20,000 40,000 60,000 80,000 100,000 0.51 0.45 overall hydrogen production efficiency 0.5 adiabatic, ASR 1.25 isothermal, ASR 1.25 adiabatic, ASR 0.25 adiabatic, ASR 0.25 0.4 adiabatic, ASR 1.25 0.49 isothermal, ASR 0.25 isothermal, ASR 0.25 isothermal, ASR 1.25 0.48 0.35 0.47 0.3 0.46 0.25 0.45 Note: the high-ASR cases shown here require ~ four times as many cells. 0.44 0.2 0 20 40 60 80 100 0 0.5 1 1.5 2 2.5 hydrogen production rate, kg/s Steam Utilization fixed utilization (imax corresponds to Vtn)
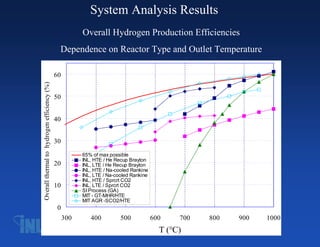
- 7. System Analysis Results Overall Hydrogen Production Efficiencies Dependence on Reactor Type and Outlet Temperature 60 Overall thermal to hydrogen efficiency (%) 50 40 30 65% of max possible INL, HTE / He Recup Brayton 20 INL, LTE / He Recup Brayton INL, HTE / Na-cooled Rankine INL, LTE / Na-cooled Rankine INL, HTE / Sprcrt CO2 10 INL, LTE / Sprcrt CO2 SI Process (GA) MIT - GT-MHR/HTE MIT AGR -SCO2/HTE 0 300 400 500 600 700 800 900 1000 T (°C)
- 8. HTE Experimental Program INL High-temperature electrolysis laboratory Integrated Laboratory Scale Small-scale experiments Facility (15 kW)
- 9. HTE Experimental Program Schematic of single-cell electrolysis test apparatus
- 10. HTE Experimental Program Exploded view of Ceramatec electrolysis stack components
- 11. HTE Experimental Program Cell Performance Characterization: Polarization curves Button cell stack -1.6 0.2 1.5 Q = 140 sccm s, Ar 10-3 10-5 theoretical open-cell potentials Q = 40.1 sccm s, H2 25-2 1.4 0 10-4 cell potential power density 25-1 per-cell operating voltage, V -1.4 -0.1 10-2 1.3 cell power density, p (W/cm ) sweep Tfrn(C) Tdp,i(C) 10-1 cell potential, E (V) 1 800 25.4 2 850 25.6 1.2 3 800 34.3 -1.2 4 850 34.4 -0.4 5 800 47.2 1.1 6 850 47.9 sweep # sccm N2 sccm H2 Tdp, i (C) Tf (C) 10-1 1011 205 48.5 800 1 10-2 2017 411 70.4 800 2 -1 E1 p1 -0.7 10-3 1017 410 83.8 800 E2 p2 10-4 2018 411 82.9 800 E3 p3 E4 p4 0.9 10-5 2018 411 83.2 830 E5 p5 25-1 2013 513 83.8 800 E6 p6 25-2 2013 513 83.4 830 electrolysis mode fuel cell mode 0.8 -0.8 -1 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 -0.6 -0.4 -0.2 0 0.2 2 2 current density, i ( A/cm ) current density, i (A/cm ) 20 Inlet CO 2 H Outlet gas composition as a function 15 Inlet H 2 2 Mole % (Dry Basis) of current density for co-electrolysis CO experiments, 10-cell stack 10 5 CO 2 Inlet CO 0 0 2 4 6 8 10 12 14 Electrolysis Current (A)
- 12. HTE Experimental Program Cell and Stack Performance Degradation Area-specific resistance vs time over ~ 1000 hrs 2.4 1.4 1.2 2 2 ASR, Ohm cm 1 ASR 1.6 0.8 increased furnace temperature from 800 C to 830 C 1.2 0.6 OCV check 0.4 0.8 0.2 0 200 400 600 800 1000 1200 0 200 400 600 800 1000 elapsed time, hr elapsed time, hrs Single button cell Stack
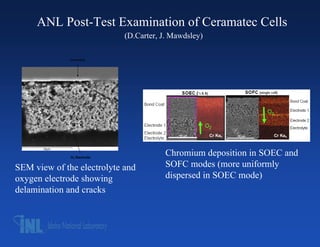
- 13. ANL Post-Test Examination of Ceramatec Cells (D.Carter, J. Mawdsley) Electrolyte O2 Electrode Chromium deposition in SOEC and SEM view of the electrolyte and SOFC modes (more uniformly oxygen electrode showing dispersed in SOEC mode) delamination and cracks
- 14. ANL Post-Test Examination of Ceramatec Cells (D.Carter, J. Mawdsley) Silica capping layer on H2-electrode Si is carried by steam from the Si-bearing seal; can also originate from interconnect plate
- 15. HTE Experimental Program Demonstration and Scale-Up: Integrated Laboratory Scale Facility Exploded view of heat exchanger, base manifold unit, and four-stack electrolysis unit ILS modules, mounted in hot zone
- 16. HTE Experimental Program Integrated Laboratory Scale Facility ILS hydrogen production rate time history Initial production rate in excess of 5 m3/hr, followed by serious degradation, some of which was related to BoP issues
- 17. Ceramatec Post-Test Examination of ILS Cells Cell and interconnect surfaces from the oxygen electrode side of ILS Cell, showing delamination electrolyte Oxygen electrode
- 18. HTE Experimental Program Performance Improvement: Single-cell test stand (electrode-supported cells) Exploded view Assembly view Photo
- 19. INL SOEC Degradation Workshop âĒ INL organized a workshop titled âDegradation in Solid Oxide Electrolysis Cells and Strategies for its Mitigation,â during the 2008 Fuel Cell Seminar & Exposition in Phoenix, AZ on October 27, 2008. âĒ The workshop was attended by researchers from academia, national laboratories, industry, several DOE representatives, and a few researchers from Japan and Germany.
- 20. Summary of INL Workshop Discussion on SOEC Degradation Mechanisms Electrodes - oxygen electrode delamination - associated with oxygen evolution in SOEC mode - possible buildup of high pressures in closed porosity Redox cycling (can lead to electrode instability) - morphology change (coarsening), reducing effective surface area of tpb region - deactivation due to contaminant transport and deposition - chromia and silicate transport and cathode poisoning (enhanced in high-steam environment) Electrolytes - Phase change in electrolyte materials with aging - Electrolytes must be fully stabilized (mechanical strength) Interconnects and seals - corrosion and non-conducting scale formation (chromia, alumina, silica), spallation in metallic interconnects, reaction with sealing glasses - Leakage from edge seals or cracked cells => hot spots
- 21. Mitigation Strategies 1. protective coatings (e.g., Co, Mn spinels) and surface treatments on interconnects â provides a barrier to inward oxygen and outward Cr diffusion 2. rare-earth surface treatments on interconnects â promote development of a stable conductive oxide scale 3. fabrication techniques, materials, operating conditions (e.g., flow distributions, current density, utilization, steam content,âĶ) 4. cell design, fabrication, materials 5. Use fully stabilized mixture, add ceria or alumina 6. Improved seals, CTE match, all-ceramic cells and stacks
- 22. Selected Additional Comments from INL Workshop Minh (GE) Oxygen Electrodes âĒ Performance: LSCF > LSF > LSM/YSZ âĒ Performance stability: LSCF and LSF have shown better performance stability than LSM/YSZ âĒ Degradation of LSCF electrode - similar in fuel cell, electrolysis, and cyclic modes, perhaps enhanced degradation in electrolysis mode âĒ Mixed conducting oxygen electrodes â better performance and stability SOEC Stacks âĒ Degradation rate 0.2-0.3 ohm-cm2/1000 h âĒ Delamination and elemental migration observed at oxygen electrode interfaces âĒ Causes for observed degradation unclear - need to be identified
- 23. Selected Additional Comments from INL Workshop (cont) Steinberger (Forschungszentrum JÞlich) Types of Degradation Phenomena âĒ Baseline degradation (continuous, steady) - Initialization phase (sintering, saturation) - constant slope phase - progressive degradation phase (EoL) âĒ degradation associated with transients - thermal cycle - redox cycle âĒ degradation after incidents (failures) - malfunction of BoP components - malfunction of control - external influence (shock, grid outage etc.)
- 24. Selected Additional Comments from INL Workshop (cont) Tang (Versa Power) Improved Cell Investigation âĒ Demonstrated significant improvement from baseline TSC2 cells âĒ Completed 3000 hours SOEC/SOFC testing âĒ Degradation rate of 39 mV/1000 hours (3 ~ 4%) Degradation Mechanism Study Indicated âĒ Combined SOEC/SOFC operation has significant higher (2x to 10x) degradation rate compare to SOFC only operation âĒ Degradations from SOEC and SOFC are symmetrical âĒ Major cause of degradation (>90%) is the cell âĒ Interconnect degradation is less than 10%
- 25. Selected Additional Comments from INL Workshop (cont) Singh (PNNL/UConn) Bi-polar corrosion of interconnects âĒ Corrosion studies need to include both reducing and oxidizing environments on either side of interconnects Glass seals âĒ Reactions with metallic interconnects to form chromates Hydrogen Electrode poisoning by Si
- 26. Conclusions and Research Plans âĒ System analysis results indicate excellent potential for large-scale hydrogen production based on HTE âĒ Good initial and long-term cell performance is critical to achieve competitive hydrogen production costs âĒ INL HTE experimental program is now focused on cell and stack performance issues: â Development of improved cell compositions (with Ceramatec) â Evaluation of advanced electrode-supported cells â Demonstration of stable long-term performance
- 27. More Information is available in numerous publications from our group! Thank You!