Drug accountability an important aspect of clinical research
- 1. Drug Accountability: An Important Aspect of Clinical Research
- 2. Introduction Drug accountability is an interesting topic related to clinical research, both for the CRAs and for the clinical research sites. Even though drug accountability isnŌĆÖt a task that should be performed by the CRA, he or she is still responsible for monitoring and making sure that the site is correctly performing every task related to this field. The topic of drug accountability is especially important in regards to quality data as well as for patient safety. For this reason, weŌĆÖll give you an in-depth explanation of everything that drug accountability entails. 2
- 3. www.trialjoin.com What is an IP? In clinical research, IP stands for Investigational Product. The investigational product is the product thatŌĆÖs being tested - it can be either the actual active ingredient or a placebo (depending on the nature of the study). 3
- 4. www.trialjoin.com IP (Drug) Accountability Steps 4
- 5. Receiving the Study Drug and IVRS/IWRS Placing the IP in a Locked Cabinet/Storage The drug accountability process starts even before randomization. More specifically, it starts as soon as the site receives the IP. Once the drugs are received, the site coordinator has to log in to IVRS (Interactive Voice Response System) or IWRS (Interactive Web Response System), take the shipment document, and manually check if all the units stated in the document are actually in the delivered box (bottles, etc.) and that they arenŌĆÖt damaged. Step 1 Step 2 www.trialjoin.com Every research site should have a locked cabinet with the appropriate temperature adjustments for keeping the investigational drug. So, after making sure that all the drugs delivered, place them in your locked cabinet. 5
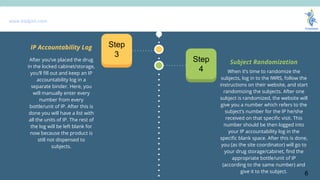
- 6. www.trialjoin.com When itŌĆÖs time to randomize the subjects, log in to the IWRS, follow the instructions on their website, and start randomizing the subjects. After one subject is randomized, the website will give you a number which refers to the subjectŌĆÖs number for the IP he/she received on that specific visit. This number should be then logged into your IP accountability log in the specific blank space. After this is done, you (as the site coordinator) will go to your drug storage/cabinet, find the appropriate bottle/unit of IP (according to the same number) and give it to the subject. Subject RandomizationStep 4 IP Accountability Log Step 3 After youŌĆÖve placed the drug in the locked cabinet/storage, youŌĆÖll fill out and keep an IP accountability log in a separate binder. Here, you will manually enter every number from every bottle/unit of IP. After this is done you will have a list with all the units of IP. The rest of the log will be left blank for now because the product is still not dispensed to subjects. 6
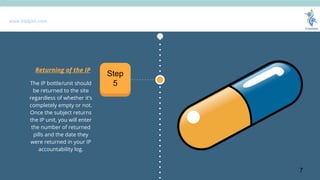
- 7. www.trialjoin.com The IP bottle/unit should be returned to the site regardless of whether itŌĆÖs completely empty or not. Once the subject returns the IP unit, you will enter the number of returned pills and the date they were returned in your IP accountability log. Returning of the IP Step 5 7
- 8. 01 02 06 07 03 04 0805 IP number of every single unit/bottle Date when the IP is received Date when the IP is assigned to a subject Which IP is assigned to which subject How many pills were assigned to each subject Date of return of the IP How many pills/units of IP were returned IP thatŌĆÖs returned to the sponsor/CRO at the end of the study and date 8 www.trialjoin.com A filled out IP accountability log should give you the following information:
- 9. The Importance of Logging Data On Time Maintaining and entering data in the IP log should always be done regularly and on time. Many coordinators forget to enter this data and when this happens, it can really quickly become a huge mess. After some time has passed, you wonŌĆÖt remember which IP is dispensed to which subject. This will make it really hard to keep track of the IP. For this reason, itŌĆÖs important to log in this data anytime someone takes or returns an IP. www.trialjoin.com 9
- 10. Close-Out Visit When the study ends, itŌĆÖs time for a close-out visit. On this visit, all your IP should be accounted for. You should have a completely filled out IP accountability log, returned (opened) bottles of used drugs, and unused (closed) drug units that werenŌĆÖt assigned to any subjects. The returned (used) units of IP and the unused ones will then be shipped back to the sponsor or CRO, and in the IP log you will fill out the appropriate field for this. www.trialjoin.com 10
- 11. www.trialjoin.com CRAs and Drug Accountability As we mentioned before, the CRA is only the monitor of the study. This means that he or she is not responsible for any of the steps explained above. The CRA will be there to only monitor that the site is doing this drug accountability and that theyŌĆÖre doing it correctly and accurately. One of the most important tasks of a CRA when it comes to drug accountability is to make sure that the IP log is constantly and properly maintained and kept up-to-date. After the study ends, on the close-out visit, the CRA (monitor) will ship the used and unused units of IP back to the sponsor or the CRO. 11
- 12. FDA warning letters for drug accountability failures Usually, the FDA will come for a drug accountability inspection at your site. Here, they will check and control if all of your drug accountability processes and activities are accurate. If they see that the site fails to comply with all drug accountability rules and regulations, they will send you a warning letter. www.trialjoin.com 12
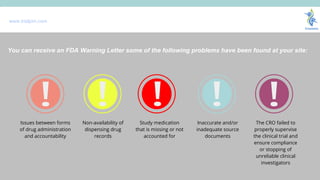
- 13. www.trialjoin.com Records of incomplete and/or incorrect distribution An adequate inventory of drugs was not maintained so now records do not identify recipients of a particular batch of the drug ThereŌĆÖs a lack of drug accountability registries in relation to drug transfer between sites Not keeping records of drug distribution during the study Shipping invoices and dispensing records that in comparison show more drugs being administered than originally received You can receive an FDA Warning Letter some of the following problems have been found at your site:
- 14. www.trialjoin.com Issues between forms of drug administration and accountability Non-availability of dispensing drug records Study medication that is missing or not accounted for Inaccurate and/or inadequate source documents The CRO failed to properly supervise the clinical trial and ensure compliance or stopping of unreliable clinical investigators You can receive an FDA Warning Letter some of the following problems have been found at your site:
- 15. www.trialjoin.com CONCLUSION To sum up, drug accountability is an important field in clinical research that will allow you to properly keep track of every single unit of IP. By doing this task correctly and promptly, you will manage to save yourself a lot of time during the close-out visit, avoid confusion, and improve data integrity and quality. Furthermore, remember that drug accountability is a task that belongs to the site (usually the site coordinator), not to the CRA. The CRA will only be there to monitor and make sure that youŌĆÖre performing these tasks properly. And finally, the most important part of this whole thing is the IP accountability log. Make sure to fill this out as soon as your IP arrives, and every time a subject takes or returns the IP. Like this, you will have a clear and completely filled out accountability log which will provide you with a better outlook on the whole picture. Drug accountability is one of the most important things in clinical research since it can greatly influence the quality and integrity of study data and results. 15