E3 13-03-01
1 like662 views
This document discusses the thermodynamics of water splitting to produce hydrogen. It explains that water decomposition requires a large positive change in free energy, so exergy equal to the change in Gibbs free energy as well as thermal energy must be supplied to shift the reaction equilibrium. The standard thermodynamic properties of the water splitting reaction are provided. Direct thermal decomposition of water is not feasible at an industrial level today due to the very high temperatures required to achieve significant dissociation.
1 of 5
Download to read offline



Ad
Recommended
Lecture of thermo-chemistry and calorimetery
Lecture of thermo-chemistry and calorimeteryBILAL ABDULLAH
╠²
This document discusses various concepts in thermochemistry including:
- Thermochemistry deals with thermal changes that accompany chemical and physical transformations. It aims to determine energy absorbed or released during reactions.
- Reactions require energy to overcome activation energy barriers to start, regardless of whether they are exothermic or endothermic. Exothermic reactions release excess energy to sustain the reaction while endothermic reactions require continuous energy input.
- Heat of formation is the energy absorbed or released when forming one mole of a substance from its elements. Hess's law allows calculating reaction energies using heats of formation without direct experimentation. Calorimetry is used to measure energy changes by determining temperature changes of a reaction mixture.Test Document
Test Documentdluetgens
╠²
This document provides instructions for an experiment to determine the heat of neutralization (ΔH) for acid-base reactions using calorimetry. It discusses key concepts like enthalpy, Hess' Law, and heat capacity. The procedure involves first calibrating thermometers and determining the heat capacity of the calorimeter. Then neutralization reactions between acids and bases are carried out in the calorimeter, and the temperature change is used to calculate the heat of reaction (ΔH) based on the calorimeter's heat capacity.C13 enthalpy change
C13 enthalpy changedean dundas
╠²
The document discusses enthalpy changes and exothermic and endothermic reactions. It defines exothermic reactions as reactions where heat energy is given out to the surroundings, while endothermic reactions absorb heat from the surroundings. It explains that the enthalpy change (ΔH) is negative for exothermic reactions and positive for endothermic reactions. The document also discusses how bond breaking requires energy input and is endothermic, while bond forming releases energy and is exothermic.Enthalpy
EnthalpyABDUL RAZZAQ SHAHID
╠²
Enthalpy is an important concept in thermochemistry that represents the heat transferred during a chemical reaction under constant pressure. It can be used to determine if a reaction is exothermic or endothermic based on the sign of ΔH. Hess's law states that the enthalpy change of a reaction is independent of the pathway and can be calculated from the enthalpies of the steps. Properties of enthalpy include it being a state function, extensive property, and dependent on the states of the reactants and products.Enthalpy
EnthalpyGandaki Boarding School,Lamachaur-16 Pokhara, Nepal
╠²
Enthalpy is a thermodynamic quantity equivalent to the total heat content of a system. Enthalpy changes (ΔH) can be exothermic or endothermic. Exothermic reactions release energy to surroundings while endothermic reactions absorb energy from surroundings. Standard enthalpy changes are used to compare reactions under standard conditions of temperature, pressure and states. Examples of standard enthalpy changes include formation, combustion, neutralization, atomization, solution, and hydration. These changes can be determined experimentally by measuring the temperature change of a reaction.Lesson : Enthalpy and Calorimetry
Lesson : Enthalpy and CalorimetryDavid Young
╠²
This document provides an overview of exothermic and endothermic reactions, calorimetry, and how to calculate enthalpy changes. It discusses key concepts like:
1) The standard enthalpy change (ΔH°) is the enthalpy change measured under standard conditions of temperature and pressure.
2) Calorimetry can be used to determine enthalpy changes by measuring the temperature change of a reaction mixture in a calorimeter.
3) Sample problems demonstrate how to use calorimetry data like temperature changes, masses, and concentrations to calculate enthalpy changes for chemical reactions.chemistry-enthalpy power point
chemistry-enthalpy power pointShmiley3000
╠²
The document provides an agenda and learning objectives for a unit on energetics. The agenda includes reading a textbook section, completing practice problems, an introduction to energetics, a Ziploc lab on calorimetry, and a calorimetry review. The learning objectives cover recalling and applying the heat transfer equation Q=mcΔT, explaining how chemical bond energy originates from the sun, and identifying reactants and products of photosynthesis, cellular respiration, and hydrocarbon combustion. The document also provides textbook content on the law of conservation of energy, examples of exothermic and endothermic reactions, heat as a transfer of energy, and calculations involving specific heat, mass, and temperature change.Thermodynamic and food
Thermodynamic and foodMaria Isabel
╠²
Thermodynamics concepts like heat transfer, phase changes, and water activity help explain important food processing techniques. The three laws of thermodynamics state that (1) energy is conserved, (2) entropy increases over time, and (3) no process is 100% efficient. Refrigeration uses phase changes - compressing and expanding gases to lower temperatures for cooling. Water activity measures the chemical potential of water in solutions and gases in contact, helping understand phenomena like dissolving, boiling, and humidity. Thermodynamics provides insights into limits and equilibrium states in many food processing applications.12 Entropy
12 Entropyjanetra
╠²
Some endothermic reactions can occur spontaneously. Whether a reaction occurs depends on both the change in enthalpy and the change in entropy. Entropy refers to the disorder of a system, and spontaneous reactions often increase the randomness or disorder. The factors of enthalpy (H) and entropy (S) combine to give the free energy (G) of a system. For a reaction to be spontaneous, the change in free energy (ΔG) must be negative. A negative ΔG can result from an exothermic reaction (negative ΔH) or from an increase in disorder (positive ΔS).Chapter 5 lecture- Thermochemistry
Chapter 5 lecture- ThermochemistryMary Beth Smith
╠²
This document provides information about various types of energy, including kinetic energy, potential energy, heat, work, and changes in internal energy. It defines key concepts such as the first law of thermodynamics, state functions, and enthalpy. It also gives examples of how to calculate heat, temperature change, enthalpy change, and energy changes using calorimetry techniques.Entropy
EntropyPRAMODA G
╠²
The document discusses the concept of entropy. It begins by defining entropy as a measure of disorder in a system and relating it to the number of microscopic states available to a system. It then discusses different types of disorder, provides an example, and gives mathematical expressions to define and calculate entropy. The document notes the importance of entropy in geochemical thermodynamics and applications like thermobarometric models. It concludes by restating that entropy increases with heat transfer at low temperatures and decreases with heat removal, and is a key concept in understanding the direction of natural processes.Enthalpy change
Enthalpy changeguest46b063
╠²
The document discusses enthalpy change (ΔH) which is the amount of heat released or absorbed during a chemical reaction under constant pressure. ΔH is calculated as the enthalpy of products minus reactants. Standard enthalpy change (ΔH°) is measured under standard temperature and pressure conditions. Some key enthalpy terms discussed include standard enthalpy of formation (ΔHf°), enthalpy of combustion (ΔHc°), enthalpy of solution (ΔHsoln), and enthalpy of neutralization. Practice problems are provided to calculate enthalpy changes using experimental heat and temperature change data.CHEMICAL THERMODYNAMICS (SPONTANEITY AND ENTROPY)
CHEMICAL THERMODYNAMICS (SPONTANEITY AND ENTROPY)NicoleGala
╠²
The document discusses spontaneous and non-spontaneous processes in thermodynamics, emphasizing entropy changes and the Second Law of Thermodynamics, which states that the total entropy of the universe increases in spontaneous processes. It introduces key concepts such as Gibbs free energy, calculating changes in entropy for reactions, and provides examples to illustrate these principles. Additionally, it outlines the relationship between enthalpy, entropy, and spontaneity, along with specific equations and sample problems to determine spontaneity under varying conditions.2012 15 3 and 15 4
2012 15 3 and 15 4David Young
╠²
This document discusses the topics of entropy and spontaneity in chemistry. It defines entropy as a measure of disorder or randomness in a system. Reactions that increase disorder have a positive change in entropy. The standard entropy change of a reaction can be calculated from standard entropy values. A spontaneous process is one that can occur without outside intervention. The Gibbs free energy change (ΔG) determines if a reaction is spontaneous - a negative ΔG means the reaction proceeds spontaneously. Temperature can affect spontaneity through the entropy term in the ΔG equation.Thermochemistry
ThermochemistryM Ali Mohsin
╠²
Thermochemistry is the study of heat changes in chemical reactions. Exothermic reactions evolve heat while endothermic reactions absorb heat. The enthalpy of a reaction is the quantity of heat absorbed or evolved. Standard enthalpy of reaction is measured under standard conditions. Hess's law states that the enthalpy change of a reaction is the same regardless of the reaction pathway. Thermochemistry is applied in pyrometallurgy which involves processes like calcination, roasting, smelting, and refining to extract metals from ores.AP Chemistry Chapter 5 Outline
AP Chemistry Chapter 5 OutlineJane Hamze
╠²
This document summarizes key concepts in thermochemistry, including:
1) Energy can be transferred as heat or work. The SI unit of energy is the joule. Internal energy is the sum of kinetic and potential energies within a system.
2) Enthalpy is a measure of the total energy of a system at constant pressure. It can be used to determine whether chemical reactions are endothermic or exothermic.
3) Calorimetry allows the measurement of heat and enthalpy changes through experiments on chemical reactions. Hess's law states that the enthalpy change of an overall reaction is the sum of enthalpy changes for individual steps.Spontaneity entropy___free_energy
Spontaneity entropy___free_energystarlanter
╠²
This document discusses spontaneity, entropy, and free energy in thermodynamics. It explains that spontaneous processes are driven by an increase in entropy of the universe according to the second law of thermodynamics. Entropy is a measure of disorder and randomness, and spontaneous reactions favor a higher entropy state. The free energy change of a reaction can predict spontaneity, with a negative value corresponding to a spontaneous process. Temperature affects spontaneity by influencing the entropy changes of both the system and surroundings.New microsoft office word document
New microsoft office word documentHina Ghazali
╠²
The document discusses several factors that can affect the rate of a chemical reaction:
1) Surface area - Increasing the surface area of a solid reactant increases the reaction rate by providing more collision sites.
2) Concentration - Increasing the concentration of a reactant increases the number of collisions between reacting particles and thus increases the reaction rate.
3) Temperature - Raising the temperature increases the kinetic energy of reacting particles, allowing more collisions with sufficient energy to result in reaction. This generally causes reaction rates to increase exponentially with temperature increases.
4) Catalysts - Catalysts provide an alternative reaction pathway requiring lower activation energy, allowing more collisions to result in reaction. This increases the reaction3 Enthalpy
3 Enthalpyjanetra
╠²
The document discusses key concepts in thermodynamics including:
1) The first law of thermodynamics states that energy cannot be created or destroyed, only changed from one form to another.
2) Enthalpy is a measurement of the total energy of a system at constant pressure.
3) Chemical reactions can be exothermic or endothermic depending on whether energy is released or absorbed.Lecture 18.4- Free Energy
Lecture 18.4- Free EnergyMary Beth Smith
╠²
This document discusses spontaneous and nonspontaneous reactions. It explains that a spontaneous reaction favors the formation of products under specified conditions, while a nonspontaneous reaction does not favor the formation of products under the same conditions. It provides the example that photosynthesis is a nonspontaneous reaction that requires an input of energy. The document also discusses entropy, enthalpy, and Gibbs free energy in the context of spontaneous reactions.What is enthalpy
What is enthalpyDr Robert Craig PhD
╠²
The document discusses the concept of enthalpy as it relates to heat exchange in chemical reactions, detailing both exothermic and endothermic processes. It explains the use of calorimeters, specifically coffee cup and bomb calorimeters, for measuring heat flow, including the necessary equations for calculating changes in enthalpy. Additionally, it provides experimental procedures and examples for students to understand heat flow and enthalpy through various chemical reactions.Entropy change during thermodynamic process
Entropy change during thermodynamic processPreetshah1212
╠²
This document discusses entropy and the laws of thermodynamics. It covers:
1) How entropy changes during different thermodynamic processes like constant volume, pressure, temperature, and adiabatic processes.
2) How entropy changes for different phases of pure substances like ice heating and melting.
3) The third law of thermodynamics which states that the entropy of a pure crystalline substance is zero at absolute zero temperature, providing an absolute reference point for determining entropy.Bioenergetics
BioenergeticsRevathi Gnanavelou
╠²
This document discusses bioenergetics and biochemical thermodynamics. It begins with an introduction to bioenergetics as the study of energy transformations that take place in living organisms. Energy is present in different forms and can be interconverted, with some forms being used to do work. Key concepts discussed include ATP as the "energy currency" of cells, oxidative phosphorylation, and the laws of thermodynamics. The document also covers topics like exergonic and endergonic reactions, Gibbs free energy, redox potentials, and high-energy compounds that store and release chemical energy like ATP.Fundamentals of Thermo-Chemistry
Fundamentals of Thermo-ChemistryRuchi Pandey
╠²
Thermochemistry is the study of heat changes in chemical reactions. It introduces various concepts including the first law of thermodynamics which states that energy cannot be created or destroyed. Enthalpy is a state function that is used to quantify heat flow during constant pressure processes. The enthalpy change of a reaction indicates whether it is exothermic or endothermic. Hess's law states that the enthalpy change of an overall reaction is equal to the sum of enthalpy changes of individual steps.Chapter 19 Lecture- Thermodynamics
Chapter 19 Lecture- ThermodynamicsMary Beth Smith
╠²
The document discusses key concepts from chemical thermodynamics including the first and second laws of thermodynamics, spontaneous and irreversible processes, entropy, and entropy on the molecular scale. It explains that entropy is a measure of disorder and randomness in a system and can be calculated using the equation S = k lnW, where W is the number of microstates corresponding to a given macrostate. The second law of thermodynamics states that the entropy of the universe increases for spontaneous processes.Thermochemistry Presentation
Thermochemistry PresentationQ M Muktadir Neal
╠²
The document discusses thermochemistry, which is the study of energy and heat in chemical reactions and physical transformations, emphasizing concepts like exothermic and endothermic reactions. It outlines different types of heat reactions, including heat of formation, decomposition, combustion, and neutralization, along with HessŌĆÖs law, stating that total enthalpy change remains consistent regardless of the reaction path. Graphical representations illustrate energy changes for these reactions, detailing how energy is absorbed or released during chemical processes.Thermochemistry
ThermochemistryShivang258
╠²
Thermochemistry is the branch of chemistry that deals with the heat absorbed or released during chemical reactions and physical transformations. It helps determine whether reactions are spontaneous or non-spontaneous by combining concepts of thermodynamics and energy in chemical bonds. Important concepts in thermochemistry include heat capacity, enthalpy, entropy, free energy, heat of combustion, and heat of formation. Thermochemistry has applications in daily life such as vehicles, refrigeration, air conditioning, power plants, and renewable energy.Fuel cell thermodynamics (2)
Fuel cell thermodynamics (2)EzhilmaranMurugesan
╠²
The document discusses the thermodynamics of fuel cells. It explains that thermodynamics is essential for understanding fuel cell performance as fuel cells convert chemical energy to electrical energy. The key thermodynamic concepts covered include entropy, enthalpy, Gibbs free energy, and how they relate to the maximum reversible voltage and efficiency of hydrogen fuel cells. Irreversible losses that decrease the actual voltage from the theoretical maximum are also discussed.Energy balance.pdf
Energy balance.pdfZeenathulFaridaAbdul1
╠²
This document discusses the thermodynamic and electrochemical principles of fuel cells. It begins by describing the basic electrochemical reactions that occur in different types of fuel cells using hydrogen, carbon monoxide, methane, and other fuels. It then explains how the ideal performance of a fuel cell can be represented by its Nernst potential and equations. The document shows how factors like temperature, pressure, and reactant concentrations affect the ideal potential. It concludes by noting that the actual potential of a fuel cell is lower than the ideal potential due to various irreversible losses during operation.More Related Content
What's hot (19)
12 Entropy
12 Entropyjanetra
╠²
Some endothermic reactions can occur spontaneously. Whether a reaction occurs depends on both the change in enthalpy and the change in entropy. Entropy refers to the disorder of a system, and spontaneous reactions often increase the randomness or disorder. The factors of enthalpy (H) and entropy (S) combine to give the free energy (G) of a system. For a reaction to be spontaneous, the change in free energy (ΔG) must be negative. A negative ΔG can result from an exothermic reaction (negative ΔH) or from an increase in disorder (positive ΔS).Chapter 5 lecture- Thermochemistry
Chapter 5 lecture- ThermochemistryMary Beth Smith
╠²
This document provides information about various types of energy, including kinetic energy, potential energy, heat, work, and changes in internal energy. It defines key concepts such as the first law of thermodynamics, state functions, and enthalpy. It also gives examples of how to calculate heat, temperature change, enthalpy change, and energy changes using calorimetry techniques.Entropy
EntropyPRAMODA G
╠²
The document discusses the concept of entropy. It begins by defining entropy as a measure of disorder in a system and relating it to the number of microscopic states available to a system. It then discusses different types of disorder, provides an example, and gives mathematical expressions to define and calculate entropy. The document notes the importance of entropy in geochemical thermodynamics and applications like thermobarometric models. It concludes by restating that entropy increases with heat transfer at low temperatures and decreases with heat removal, and is a key concept in understanding the direction of natural processes.Enthalpy change
Enthalpy changeguest46b063
╠²
The document discusses enthalpy change (ΔH) which is the amount of heat released or absorbed during a chemical reaction under constant pressure. ΔH is calculated as the enthalpy of products minus reactants. Standard enthalpy change (ΔH°) is measured under standard temperature and pressure conditions. Some key enthalpy terms discussed include standard enthalpy of formation (ΔHf°), enthalpy of combustion (ΔHc°), enthalpy of solution (ΔHsoln), and enthalpy of neutralization. Practice problems are provided to calculate enthalpy changes using experimental heat and temperature change data.CHEMICAL THERMODYNAMICS (SPONTANEITY AND ENTROPY)
CHEMICAL THERMODYNAMICS (SPONTANEITY AND ENTROPY)NicoleGala
╠²
The document discusses spontaneous and non-spontaneous processes in thermodynamics, emphasizing entropy changes and the Second Law of Thermodynamics, which states that the total entropy of the universe increases in spontaneous processes. It introduces key concepts such as Gibbs free energy, calculating changes in entropy for reactions, and provides examples to illustrate these principles. Additionally, it outlines the relationship between enthalpy, entropy, and spontaneity, along with specific equations and sample problems to determine spontaneity under varying conditions.2012 15 3 and 15 4
2012 15 3 and 15 4David Young
╠²
This document discusses the topics of entropy and spontaneity in chemistry. It defines entropy as a measure of disorder or randomness in a system. Reactions that increase disorder have a positive change in entropy. The standard entropy change of a reaction can be calculated from standard entropy values. A spontaneous process is one that can occur without outside intervention. The Gibbs free energy change (ΔG) determines if a reaction is spontaneous - a negative ΔG means the reaction proceeds spontaneously. Temperature can affect spontaneity through the entropy term in the ΔG equation.Thermochemistry
ThermochemistryM Ali Mohsin
╠²
Thermochemistry is the study of heat changes in chemical reactions. Exothermic reactions evolve heat while endothermic reactions absorb heat. The enthalpy of a reaction is the quantity of heat absorbed or evolved. Standard enthalpy of reaction is measured under standard conditions. Hess's law states that the enthalpy change of a reaction is the same regardless of the reaction pathway. Thermochemistry is applied in pyrometallurgy which involves processes like calcination, roasting, smelting, and refining to extract metals from ores.AP Chemistry Chapter 5 Outline
AP Chemistry Chapter 5 OutlineJane Hamze
╠²
This document summarizes key concepts in thermochemistry, including:
1) Energy can be transferred as heat or work. The SI unit of energy is the joule. Internal energy is the sum of kinetic and potential energies within a system.
2) Enthalpy is a measure of the total energy of a system at constant pressure. It can be used to determine whether chemical reactions are endothermic or exothermic.
3) Calorimetry allows the measurement of heat and enthalpy changes through experiments on chemical reactions. Hess's law states that the enthalpy change of an overall reaction is the sum of enthalpy changes for individual steps.Spontaneity entropy___free_energy
Spontaneity entropy___free_energystarlanter
╠²
This document discusses spontaneity, entropy, and free energy in thermodynamics. It explains that spontaneous processes are driven by an increase in entropy of the universe according to the second law of thermodynamics. Entropy is a measure of disorder and randomness, and spontaneous reactions favor a higher entropy state. The free energy change of a reaction can predict spontaneity, with a negative value corresponding to a spontaneous process. Temperature affects spontaneity by influencing the entropy changes of both the system and surroundings.New microsoft office word document
New microsoft office word documentHina Ghazali
╠²
The document discusses several factors that can affect the rate of a chemical reaction:
1) Surface area - Increasing the surface area of a solid reactant increases the reaction rate by providing more collision sites.
2) Concentration - Increasing the concentration of a reactant increases the number of collisions between reacting particles and thus increases the reaction rate.
3) Temperature - Raising the temperature increases the kinetic energy of reacting particles, allowing more collisions with sufficient energy to result in reaction. This generally causes reaction rates to increase exponentially with temperature increases.
4) Catalysts - Catalysts provide an alternative reaction pathway requiring lower activation energy, allowing more collisions to result in reaction. This increases the reaction3 Enthalpy
3 Enthalpyjanetra
╠²
The document discusses key concepts in thermodynamics including:
1) The first law of thermodynamics states that energy cannot be created or destroyed, only changed from one form to another.
2) Enthalpy is a measurement of the total energy of a system at constant pressure.
3) Chemical reactions can be exothermic or endothermic depending on whether energy is released or absorbed.Lecture 18.4- Free Energy
Lecture 18.4- Free EnergyMary Beth Smith
╠²
This document discusses spontaneous and nonspontaneous reactions. It explains that a spontaneous reaction favors the formation of products under specified conditions, while a nonspontaneous reaction does not favor the formation of products under the same conditions. It provides the example that photosynthesis is a nonspontaneous reaction that requires an input of energy. The document also discusses entropy, enthalpy, and Gibbs free energy in the context of spontaneous reactions.What is enthalpy
What is enthalpyDr Robert Craig PhD
╠²
The document discusses the concept of enthalpy as it relates to heat exchange in chemical reactions, detailing both exothermic and endothermic processes. It explains the use of calorimeters, specifically coffee cup and bomb calorimeters, for measuring heat flow, including the necessary equations for calculating changes in enthalpy. Additionally, it provides experimental procedures and examples for students to understand heat flow and enthalpy through various chemical reactions.Entropy change during thermodynamic process
Entropy change during thermodynamic processPreetshah1212
╠²
This document discusses entropy and the laws of thermodynamics. It covers:
1) How entropy changes during different thermodynamic processes like constant volume, pressure, temperature, and adiabatic processes.
2) How entropy changes for different phases of pure substances like ice heating and melting.
3) The third law of thermodynamics which states that the entropy of a pure crystalline substance is zero at absolute zero temperature, providing an absolute reference point for determining entropy.Bioenergetics
BioenergeticsRevathi Gnanavelou
╠²
This document discusses bioenergetics and biochemical thermodynamics. It begins with an introduction to bioenergetics as the study of energy transformations that take place in living organisms. Energy is present in different forms and can be interconverted, with some forms being used to do work. Key concepts discussed include ATP as the "energy currency" of cells, oxidative phosphorylation, and the laws of thermodynamics. The document also covers topics like exergonic and endergonic reactions, Gibbs free energy, redox potentials, and high-energy compounds that store and release chemical energy like ATP.Fundamentals of Thermo-Chemistry
Fundamentals of Thermo-ChemistryRuchi Pandey
╠²
Thermochemistry is the study of heat changes in chemical reactions. It introduces various concepts including the first law of thermodynamics which states that energy cannot be created or destroyed. Enthalpy is a state function that is used to quantify heat flow during constant pressure processes. The enthalpy change of a reaction indicates whether it is exothermic or endothermic. Hess's law states that the enthalpy change of an overall reaction is equal to the sum of enthalpy changes of individual steps.Chapter 19 Lecture- Thermodynamics
Chapter 19 Lecture- ThermodynamicsMary Beth Smith
╠²
The document discusses key concepts from chemical thermodynamics including the first and second laws of thermodynamics, spontaneous and irreversible processes, entropy, and entropy on the molecular scale. It explains that entropy is a measure of disorder and randomness in a system and can be calculated using the equation S = k lnW, where W is the number of microstates corresponding to a given macrostate. The second law of thermodynamics states that the entropy of the universe increases for spontaneous processes.Thermochemistry Presentation
Thermochemistry PresentationQ M Muktadir Neal
╠²
The document discusses thermochemistry, which is the study of energy and heat in chemical reactions and physical transformations, emphasizing concepts like exothermic and endothermic reactions. It outlines different types of heat reactions, including heat of formation, decomposition, combustion, and neutralization, along with HessŌĆÖs law, stating that total enthalpy change remains consistent regardless of the reaction path. Graphical representations illustrate energy changes for these reactions, detailing how energy is absorbed or released during chemical processes.Thermochemistry
ThermochemistryShivang258
╠²
Thermochemistry is the branch of chemistry that deals with the heat absorbed or released during chemical reactions and physical transformations. It helps determine whether reactions are spontaneous or non-spontaneous by combining concepts of thermodynamics and energy in chemical bonds. Important concepts in thermochemistry include heat capacity, enthalpy, entropy, free energy, heat of combustion, and heat of formation. Thermochemistry has applications in daily life such as vehicles, refrigeration, air conditioning, power plants, and renewable energy.Similar to E3 13-03-01 (20)
Fuel cell thermodynamics (2)
Fuel cell thermodynamics (2)EzhilmaranMurugesan
╠²
The document discusses the thermodynamics of fuel cells. It explains that thermodynamics is essential for understanding fuel cell performance as fuel cells convert chemical energy to electrical energy. The key thermodynamic concepts covered include entropy, enthalpy, Gibbs free energy, and how they relate to the maximum reversible voltage and efficiency of hydrogen fuel cells. Irreversible losses that decrease the actual voltage from the theoretical maximum are also discussed.Energy balance.pdf
Energy balance.pdfZeenathulFaridaAbdul1
╠²
This document discusses the thermodynamic and electrochemical principles of fuel cells. It begins by describing the basic electrochemical reactions that occur in different types of fuel cells using hydrogen, carbon monoxide, methane, and other fuels. It then explains how the ideal performance of a fuel cell can be represented by its Nernst potential and equations. The document shows how factors like temperature, pressure, and reactant concentrations affect the ideal potential. It concludes by noting that the actual potential of a fuel cell is lower than the ideal potential due to various irreversible losses during operation.Modelling and simulation approach.pdf
Modelling and simulation approach.pdfAhmed Samir
╠²
This document provides an overview of modeling and simulation approaches for an alkaline water electrolyzer. It describes the electrolysis process and reaction equations. A thermodynamic model is presented that calculates the reversible voltage and thermoneutral potential from changes in Gibbs free energy and enthalpy with temperature. The document also discusses sources of cell overpotential including activation, ohmic resistance, and gas bubble formation that increase the actual operating voltage above the minimum reversible value. Flow rates of hydrogen and oxygen produced are calculated from Faraday's laws using current and Faraday efficiency.A review on water electrolysis
A review on water electrolysisABDUL RAZZAQ SHAHID
╠²
1. The document provides a historical overview of water electrolysis from its discovery in 1789 to modern developments. Nicholson and Carlisle were the first to develop the technique in 1800, and by 1902 there were over 400 industrial units in operation.
2. It explains the theory behind water electrolysis, including the chemical reactions that produce hydrogen and oxygen, factors that determine minimum voltage requirements, and sources of inefficiency.
3. Various methods for producing hydrogen through water electrolysis are briefly described, including alkaline electrolysis, proton exchange membrane electrolysis, and producing hydrogen as a byproduct of chloralkali production. Advanced alkaline systems and high-pressure designs are highlighted.AP_Chem_Thermodynamics.pptx
AP_Chem_Thermodynamics.pptxMadeBramasta
╠²
Thermodynamics is the study of energy and its transformations. Thermochemistry is the subdiscipline involving chemical reactions and energy changes. The first law of thermodynamics states that energy is conserved as it transforms between forms. Energy exists in kinetic and potential forms. Kinetic energy is associated with motion, while potential energy is stored energy due to position or composition. Heat is the transfer of kinetic energy between objects of different temperatures until they reach equilibrium. Exothermic processes release heat from a system, while endothermic processes absorb heat into a system. Enthalpy changes (ΔH) quantify the heat absorbed or released by chemical reactions. Spontaneous processes are those that proceed without outside intervention, as dictated by a negative changeDeep subsea OTEC
Deep subsea OTECVicente Fachina
╠²
This document presents the deep-subsea Ocean Thermal Energy Conversion (OTEC) concept, highlighting its threefold increase in exergy efficiency compared to topside systems, potential for negative CO2 emissions, and estimated costs of about USD 600 million for a 100 MW system. The deep-subsea design minimizes environmental impact by preventing CO2 upwelling and reduces investment costs by omitting naval structures. Overall, the deep-subsea OTEC offers a more efficient and cost-effective solution for renewable energy production in suitable tropical locations.panakaj klksajdkhsuiosddfdnkajhaK UHAUISUKd
panakaj klksajdkhsuiosddfdnkajhaK UHAUISUKdpankajkumar3480
╠²
The document discusses various methods of hydrogen production from water, including electrolysis, thermochemical processes, and photoelectrochemical systems. It outlines the technical details of these methods, their efficiencies, and comparisons to other energy generation techniques, emphasizing advancements in electrolysis efficiency and potential alternative methods. Additionally, it highlights the implications of these methods for sustainable energy and hydrogen economy applications.HYDROGEN : HYDROGEN production, electolysis,photoelectrolysis
HYDROGEN : HYDROGEN production, electolysis,photoelectrolysisbrijsharma3371
╠²
The document discusses various methods for hydrogen production, emphasizing techniques such as electrolysis of water, thermal decomposition, and photo-electrolysis using solar energy. It highlights the energy efficiency and potentials of these methods while comparing them against traditional practices that involve fossil fuels. Additionally, it explores advancements in catalysts and thermochemical processes for optimizing hydrogen production sustainably.Function of state
Function of state K. Shahzad Baig
╠²
The document discusses the concept of state functions in thermodynamics, emphasizing that properties like internal energy and enthalpy depend on the state of the system rather than how that state is achieved. It explains the first law of thermodynamics and how heat and work relate to internal energy and enthalpy changes during chemical reactions. The document also details calculations for specific heat, reaction enthalpies, and phase changes, illustrating how to determine energy exchanges in various processes.Isde 6
Isde 6Alexander Decker
╠²
The document discusses an innovative method for generating hydroxy gas (a mixture of hydrogen and oxygen) through a novel electronic circuit that uses extremely low current to electrolyze water without relying on traditional electrolytes, thus improving energy efficiency. The method is based on utilizing the natural ionic dissociation of water molecules, enhanced by high-voltage pulses, to separate hydrogen and oxygen gases effectively while adhering to the laws of thermodynamics. Furthermore, the process is positioned as a potential solution for sustainable energy needs, particularly for automotive applications.Hydrogen Production ppt.pptx
Hydrogen Production ppt.pptxMdHelalHossain6
╠²
The document discusses several methods for producing hydrogen through water splitting, including:
- Steam reforming of methane, the most common current method.
- Electrolysis, where an electric current splits water into hydrogen and oxygen. More efficient variations include steam electrolysis and thermochemical electrolysis.
- Photochemical and photobiological systems use sunlight to drive the water splitting reaction.
- Thermal water splitting uses very high temperatures of around 1000┬░C.
- Gasification and biomass conversion also produce hydrogen from other feedstocks.
Low current electrolysis is discussed as a more efficient method, similar to the water splitting that occurs in photosynthesis. Producing hydrogen directly from water without electrolysis is also mentioned. OverallModeling electrolyte solutions with the extended universal quasi chemical (u...
Modeling electrolyte solutions with the extended universal quasi chemical (u...nazanin25
╠²
The document describes the extended UNIQUAC model for modeling electrolyte solutions. The extended UNIQUAC model consists of three terms: a combinatorial or entropic term, a residual or enthalpic term, and an electrostatic term from the Debye-H├╝ckel equation. The model can be used to describe solid-liquid, liquid-liquid, and vapor-liquid equilibria using UNIQUAC volume, surface area, and interaction energy parameters determined from experimental data. Thermal properties are also calculated accurately by the extended UNIQUAC model using a single set of parameters.Thermochemistry (Production of Heat).pptx
Thermochemistry (Production of Heat).pptxTamaraCarey1
╠²
The document covers key concepts in thermochemistry, detailing the first law of thermodynamics, definitions of energy forms, and the distinction between heat and thermal energy. It explains endothermic and exothermic processes, the role of enthalpy in measuring heat changes, and introduces Hess's law for calculating enthalpy changes in reactions. Additionally, it discusses entropy and Gibbs free energy in the context of spontaneous processes and thermodynamic principles.k-cycle.17
k-cycle.17Chan-Hee Koh
╠²
1) The document proposes a system that uses solar energy to recharge an entrochemical device, which spontaneously generates a thermal gradient between two solutions of differing salt concentrations.
2) A solar chimney is used to generate airflow over a "draw solution", evaporating water and reconcentrating the solution. This restored concentration gradient re-enables the entrochemical system.
3) Experimental results show thermal battery outputs of 129.3-235.3 W/m2 and internal recharge rates of 55.6-116.4 W/m2 using various salt pairs. A 6.1m tall solar chimney achieved evaporative performance of 1.213 L/day/m2, correspondingCH-2 air conditioning thermo chapter.pptx
CH-2 air conditioning thermo chapter.pptxHaylemariyamBugna
╠²
The document outlines the principles of gas-vapor mixtures in the context of thermodynamics, particularly pertaining to atmospheric air and its properties such as specific and relative humidity, dew-point temperature, and enthalpy. It also includes applications of these principles in air-conditioning processes and examples of calculations for various air mixture conditions. Additionally, it discusses the interactions between dry air and water vapor, emphasizing the significance of humidity in environmental comfort.Inorganic Chemistry: Thermochemistry
Inorganic Chemistry: ThermochemistryThivyaapriya Sambamoorthy
╠²
This document provides an introduction to thermochemistry and the key concepts of enthalpy, enthalpy change, and standard enthalpy of formation. It defines system and surroundings, and the three types of systems - open, closed, and isolated. The key points are:
- Enthalpy change (ΔH) is the difference in enthalpies between products and reactants and indicates whether a reaction is endothermic or exothermic.
- Standard enthalpy of formation (H┬░f) is the enthalpy change when 1 mole of a substance is formed from its elements under standard conditions.
- Enthalpy of combustion (H┬░c) is the enthalpy change when 1 molePEM Water Electrolysis
PEM Water ElectrolysisRichard Smith
╠²
This document summarizes proton exchange membrane water electrolysis. It discusses how water electrolysis produces hydrogen and oxygen gas through an electric energy input that splits water molecules. A proton exchange membrane electrolyzer uses bipolar plates, gas diffusion layers, a membrane electrode assembly, and electrocatalysts. The membrane allows for hydronium ion transport without electron conduction. Electrolysis provides a method for renewable energy storage and production of hydrogen as an energy carrier or for green methanol synthesis.Fuelcell
FuelcellMechanical Design Engineering
╠²
The document provides an overview of fuel cell technology. It discusses the brief history of fuel cells and the basic principles of electrolysis and how fuel cells work by reversing the electrolysis process. It describes the main components of a fuel cell and the five most common types: alkaline, molten carbonate, phosphoric acid, proton exchange membrane, and solid oxide fuel cells. The benefits of fuel cells are highlighted such as efficiency, reliability and fuel flexibility. Challenges for different fuel cell types are also summarized, for example high operating temperatures of solid oxide fuel cells can limit applications.Thermochemistry ok1294993378
Thermochemistry ok1294993378Navin Joshi
╠²
The document discusses energy changes that occur during chemical reactions. It defines exothermic and endothermic reactions, and standard enthalpy change. It provides examples of how to calculate enthalpy changes from experimental temperature change data using concepts like specific heat capacity. Hess's law, which states the enthalpy change of a reaction is equal to the sum of enthalpy changes of the steps in a reaction mechanism, is also introduced.Ad
More from ABDUL RAZZAQ SHAHID (7)
Wp 100 eberhard_future_of_south_african_coal
Wp 100 eberhard_future_of_south_african_coalABDUL RAZZAQ SHAHID
╠²
This document summarizes the challenges facing South Africa's coal industry. South Africa is a major global coal producer and exporter, but its position has slipped in recent years. The country has large reserves but faces constraints like inadequate rail infrastructure. Most coal is used domestically for electricity and liquid fuels, but there is potential to increase exports. The top coal companies have emerged from the country's historical mining interests. However, issues like environmental impacts, climate policy, and future demand uncertainty could affect the coal industry's development.Marston review of_indonesian_thermal_coal_industry(1)
Marston review of_indonesian_thermal_coal_industry(1)ABDUL RAZZAQ SHAHID
╠²
1) Indonesia is the world's largest exporter of thermal coal, exporting 165 million tons in 2007. The country's coal industry continues to grow due to rising regional and domestic demand.
2) Major Indonesian coal exporters include Bumi Resources, Adaro Resources, Banpu, and Kideco Jaya Agung, which collectively exported over 130 million tons in 2007.
3) Indonesian coal ranges from bituminous to sub-bituminous in rank and varies in qualities like ash content, heat value, and sulfur content. Most coal is transported from mines to loading facilities by truck and barge before being exported.Coal firing
Coal firingABDUL RAZZAQ SHAHID
╠²
This document provides information on coal firing systems and components supplied by Hitachi Power Europe GmbH (HPE). HPE is a technology leader in fossil-fired power plants, designing and supplying key components like pulverizers, burners, and ash removal systems. The document discusses HPE's selection criteria for furnace and firing systems, and describes the various coal mill types, burners, and other components that HPE provides to handle different coal types from hard coal to lignite. It also provides examples of power plant projects where HPE has provided these systems.Session 2 module 2 coal properties and effect on cobustion
Session 2 module 2 coal properties and effect on cobustionABDUL RAZZAQ SHAHID
╠²
This document discusses how coal properties influence boiler design and operation. Key coal properties like moisture content, ash content, volatile matter, and sulfur content affect combustion performance, mill performance, boiler efficiency, slagging, fouling, ESP performance, and the life of boiler components. The furnace design must consider factors like fuel ratio, ash loading, heat release rates, and slagging/fouling characteristics. Indian coals generally have higher ash and moisture contents compared to international coals, which impacts the design of systems like mills and ESPs. The boiler engineer must carefully evaluate these coal properties to optimize boiler performance.Fire flow design guidelines 2011
Fire flow design guidelines 2011ABDUL RAZZAQ SHAHID
╠²
This document provides guidelines for fire flow design in water reticulation systems. It outlines general design criteria such as using a 95th percentile background demand, providing a minimum of 10 L/s from one hydrant for residential and two hydrants for other areas, maintaining a positive residual pressure, providing 4 hours reserve storage, and recommended minimum pipe diameters of 100mm for low-rise residential and 150mm for other areas. It also provides guidance on managing developer requests, marking and maintaining hydrants, and reviewing relevant legislation, standards, and the expectations of the NSW Fire Brigade.Bacterial typing idse14_wm
Bacterial typing idse14_wmABDUL RAZZAQ SHAHID
╠²
The introduction of new molecular diagnostic technologies is transforming clinical microbiology testing by providing faster results compared to traditional culture-based methods. These technologies like PCR, mass spectrometry, and next-generation sequencing can provide results in hours rather than days. Earlier diagnostic information from these tests has been shown to improve patient outcomes. Many laboratories are evaluating how to incorporate molecular testing while reducing reliance on culture-based methods. These new tests are also being applied to infection control practice to help with tasks like surveillance and isolation precautions.16 4 boston_04-72_0079
16 4 boston_04-72_0079ABDUL RAZZAQ SHAHID
╠²
This document summarizes research on multi-step processes for decomposing water into hydrogen and oxygen. It discusses:
1) The thermodynamic limitations of a single-step process based on entropy and temperature considerations.
2) How a multi-step process can reduce the work requirement by varying reaction entropy changes across steps and operating different reaction groups at different temperatures.
3) The theoretical minimum work of separating reaction products based on free energy and entropy, and how this sets a lower limit than the standard free energy change of the overall reaction.
4) An analysis of the vanadium chloride four-step process as an example, finding it is less efficient than water electrolysis. The document aims to understand whyAd
E3 13-03-01
- 1. U N ESC O ŌĆō EO LSS SAM PLE C H APTER S ENERGY CARRIERS AND CONVERSION SYSTEMS ŌĆō Vol. I - Thermodynamics of Water Splitting - Atsushi Tsutsumi ┬®Encyclopedia of Life Support Systems (EOLSS) THERMODYNAMICS OF WATER SPLITTING Atsushi Tsutsumi University of Tokyo, Japan Keywords: Hydrogen production, Water splitting, Thermodynamics, Gibbs free energy, enthalpy, Exergy, Exergy rate, Electrolysis, Thermochemical water decomposition, Chemical Reaction Contents 1. Introduction: Fundamentals 2. Electrolysis of Water 3. Thermochemical Water Splitting Glossary Bibliography Biographical Sketch Summary Since the water decomposition reaction has a large positive free energy change, exergy equal to G╬ö , as well as thermal energy T S╬ö , should be supplied to shift the equilibrium of a water-splitting reaction. 1. Introduction: Fundamentals The energy required for the endothermic reaction is equal to the enthalpy change of reaction, H╬ö , and consists of two parts: a thermal energy requirement, Q╬ö , and a useful work requirement, W╬ö . In the case of a reversible chemical reaction at constant temperature and pressure the following conditions hold: W G╬ö = ŌłÆ╬ö (1) T S Q╬ö = ╬ö (2) Where G╬ö and S╬ö represent changes in Gibbs free energy and reaction entropy respectively. Thus, we write: H G T SŌłÆ╬ö = ŌłÆ╬ö ŌłÆ ╬ö (3) The equilibrium constant K is related to the Gibbs free energy change G╬ö for the chemical reaction as the follows: lnG RT K╬ö = ŌłÆ (4) With the decrease in G╬ö the equilibrium constant of chemical reaction increases. At
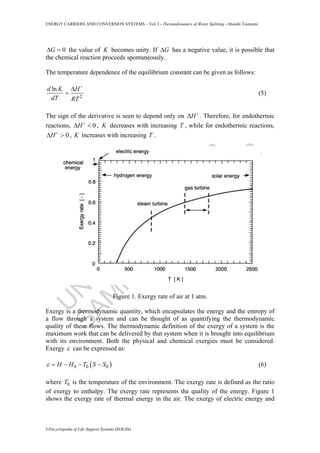
- 2. U N ESC O ŌĆō EO LSS SAM PLE C H APTER S ENERGY CARRIERS AND CONVERSION SYSTEMS ŌĆō Vol. I - Thermodynamics of Water Splitting - Atsushi Tsutsumi ┬®Encyclopedia of Life Support Systems (EOLSS) 0G╬ö = the value of K becomes unity. If G╬ö has a negative value, it is possible that the chemical reaction proceeds spontaneously. The temperature dependence of the equilibrium constant can be given as follows: 2 lnd K H dT RT ╬ö = (5) The sign of the derivative is seen to depend only on H╬ö . Therefore, for endothermic reactions, 0H╬ö < , K decreases with increasing T , while for endothermic reactions, 0H╬ö > , K increases with increasing T . Figure 1. Exergy rate of air at 1 atm. Exergy is a thermodynamic quantity, which encapsulates the energy and the entropy of a flow through a system and can be thought of as quantifying the thermodynamic quality of these flows. The thermodynamic definition of the exergy of a system is the maximum work that can be delivered by that system when it is brought into equilibrium with its environment. Both the physical and chemical exergies must be considered. Exergy ╬Ą can be expressed as: ( )0 0 0H H T S S╬Ą = ŌłÆ ŌłÆ ŌłÆ (6) where 0T is the temperature of the environment. The exergy rate is defined as the ratio of exergy to enthalpy. The exergy rate represents the quality of the energy. Figure 1 shows the exergy rate of thermal energy in the air. The exergy of electric energy and
- 3. U N ESC O ŌĆō EO LSS SAM PLE C H APTER S ENERGY CARRIERS AND CONVERSION SYSTEMS ŌĆō Vol. I - Thermodynamics of Water Splitting - Atsushi Tsutsumi ┬®Encyclopedia of Life Support Systems (EOLSS) mechanical energy is unity, while the thermal energy at 1000 ┬░C and 2000 ┬░C has an exergy rate of 0.5 and 0.7, respectively. The exergy loss is generated throughout the process by the irreversibility. Hydrogen has a relatively low exergy rate (0.83), while the exergy rate of conventional fuels ranges from 0.92 to 0.96. Therefore, hydrogen combustion can reduce the exergy loss in an irreversible combustion process. Hydrogen is produced by direct water decomposition as: 2 2 2 1 H O H + O 2 ŌåÆ (7) Supposing that water is initially in the gas phase, the standard thermodynamic functions are as follows: 241.93 kJ/molH╬ö = (8) 228.71 kJ/molG╬ö = (9) 44.333 kJ/mol KS╬ö = Ōŗģ (10) 9.68 kJ/mol KpC╬ö = Ōŗģ (11) Figure 2. ŌłåG-T diagram of water splitting. Because of the endothermic reaction the energy that is equal to H╬ö should be provided to split water into hydrogen and oxygen; G╬ö as a useful work and T S╬ö as a thermal energy. The G T╬ö ŌłÆ diagram for the water splitting reaction is shown in Figure 2. The water decomposition reaction has a large positive free energy change and a small positive entropy change, as can be seen in Figure 2. Thus, the equilibrium for the reaction is unfavorable for hydrogen production. The water decomposition reaction is
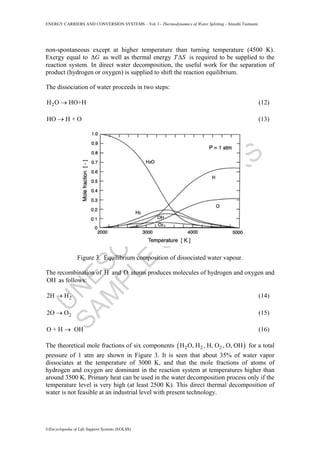
- 4. U N ESC O ŌĆō EO LSS SAM PLE C H APTER S ENERGY CARRIERS AND CONVERSION SYSTEMS ŌĆō Vol. I - Thermodynamics of Water Splitting - Atsushi Tsutsumi ┬®Encyclopedia of Life Support Systems (EOLSS) non-spontaneous except at higher temperature than turning temperature (4500 K). Exergy equal to G╬ö as well as thermal energy T S╬ö is required to be supplied to the reaction system. In direct water decomposition, the useful work for the separation of product (hydrogen or oxygen) is supplied to shift the reaction equilibrium. The dissociation of water proceeds in two steps: 2H O HO+HŌåÆ (12) HO H + OŌåÆ (13) Figure 3. Equilibrium composition of dissociated water vapour. The recombination of H and O atoms produces molecules of hydrogen and oxygen and OH as follows: 22H HŌåÆ (14) 22O OŌåÆ (15) O + H OHŌåÆ (16) The theoretical mole fractions of six components ( )2 2 2H O, H , H, O , O, OH for a total pressure of 1 atm are shown in Figure 3. It is seen that about 35% of water vapor dissociates at the temperature of 3000 K, and that the mole fractions of atoms of hydrogen and oxygen are dominant in the reaction system at temperatures higher than around 3500 K. Primary heat can be used in the water decomposition process only if the temperature level is very high (at least 2500 K). This direct thermal decomposition of water is not feasible at an industrial level with present technology.
- 5. U N ESC O ŌĆō EO LSS SAM PLE C H APTER S ENERGY CARRIERS AND CONVERSION SYSTEMS ŌĆō Vol. I - Thermodynamics of Water Splitting - Atsushi Tsutsumi ┬®Encyclopedia of Life Support Systems (EOLSS) - - - TO ACCESS ALL THE 10 PAGES OF THIS CHAPTER, Visit: http://www.eolss.net/Eolss-sampleAllChapter.aspx Bibliography Kyle B. G. (1984). Chemical and Processes Thermodynamics, 512 pp. Englewood Cliffs, NJ: Prentice- Hall. Ohta T. (1994). Energy Technology, Sources, Systems and Frontier Conversions, 235 pp. Oxford: Pergamon Press. Yoshida K., ed. Energy Engineering, 213 pp., Tokyo: Kyoritsu Syuppan. Biographical Sketch Atsushi Tsutsumi, born November 2, 1956, in Japan, is an Associate Professor of Chemical System Engineering at University of Tokyo. He received his Doctorate of Engineering from the University of Tokyo in 1986. He has been active in research on innovative thermochemical energy technology and nonlinear dynamics and chaos scale-up methodology development for multi-phase reactors for the last ten years. He has published over 70 scientific publications and 100 proceedings in international journals and conferences.
