CDISC Edetek Panther CTMP introduction
- 2. AGENDA ? ? ? ? ? ? Introductions EDETEK Overview CDISC Compliance Panther CTMP? Quality Control CDISC Data Analytics and Submission Services 2
- 3. INTRODUCTION TO EDETEK Company CDISC Commitment ? ? ? ? ? ? CDISC ˇ°Gold Memberˇ± ? Member of 6 CDISC Working Groups: SDS, ADaM, CDASH, ODM, CT, Share ? Participated in CDISC Pilot ? CDISC Registered Solution Provider (RSP) for SDTM, ADaM, & Define.xml Services Quality Work Full-service CRO established in 2009 Headquarter in Princeton, NJ Branch office in Beijing ISO 9001-2008 Certified Over 50 member team with more than 30 dedicated to CDISC Services ? Partner with multiple clinics in US and 600+ sites in China ? ? ? ? ? ? ? ? Full data management services to 20 pharmaceutical companies ? Prepared hundreds of study packages ? Completed 4 NDA submission (including ISS/ISE packages) in 2013 ? Customers including largest pharmaceutical companies ? World-wide data standardization partner of HCL Study Design ¨C Protocol Development Monitoring Clinical Data/Study Management Stats and Programming CDISC Conversion/e-Submission Medical Writing Technology & Platform Provider 3
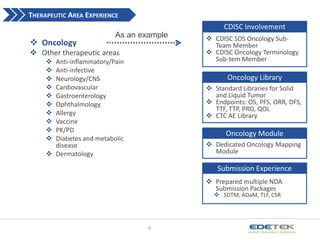
- 4. THERAPEUTIC AREA EXPERIENCE CDISC Involvement ? Oncology As an example ? Other therapeutic areas ? ? ? ? ? ? ? ? ? ? Anti-inflammatory/Pain Anti-infective Neurology/CNS Cardiovascular Gastroenterology Ophthalmology Allergy Vaccine PK/PD Diabetes and metabolic disease ? Dermatology ? CDISC SDS Oncology SubTeam Member ? CDISC Oncology Terminology Sub-tem Member Oncology Library ? Standard Libraries for Solid and Liquid Tumor ? Endpoints: OS, PFS, ORR, DFS, TTF, TTP, PRO, QOL ? CTC AE Library Oncology Module ? Dedicated Oncology Mapping Module Submission Experience ? Prepared multiple NDA Submission Packages ? SDTM, ADaM, TLF, CSR 4
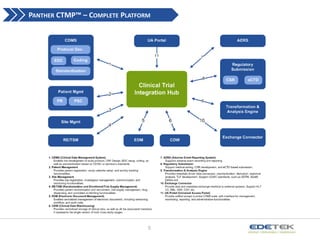
- 5. PANTHER CTMP? ¨C COMPLETE PLATFORM 5
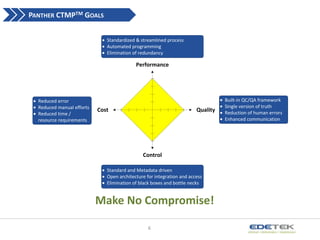
- 6. PANTHER CTMPTM GOALS Standardized & streamlined process Automated programming Elimination of redundancy Performance Reduced error Reduced manual efforts Reduced time / resource requirements Cost Quality Control Standard and Metadata driven Open architecture for integration and access Elimination of black boxes and bottle necks Make No Compromise! 6 Built-in QC/QA framework Single version of truth Reduction of human errors Enhanced communication
- 7. PANTHER CDMSTM External System Unified Access Portal Web Services CRF Design Study Administration Data Repository Forms & Items Study Management Data Capture Presentation Layout Site Management External Data Integration Standardization (SDTM) Edit Checks Investigator Management Query Management Coding Standardization Rules Patient Management Document Management Panther CDMSTM Study Protocol CDASH Sponsor Standards Edit Check Library Data Standardization Reporting Monitoring Administration 7 Coding
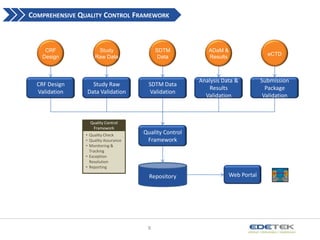
- 8. COMPREHENSIVE QUALITY CONTROL FRAMEWORK CRF Design Study Raw Data SDTM Data ADaM & Results eCTD CRF Design Validation Study Raw Data Validation SDTM Data Validation Analysis Data & Results Validation Submission Package Validation Quality Control Framework ? Quality Check ? Quality Assurance ? Monitoring & Tracking ? Exception Resolution ? Reporting Quality Control Framework Repository 8 Web Portal
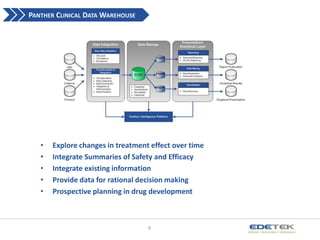
- 9. PANTHER CLINICAL DATA WAREHOUSE ? ? ? ? ? Explore changes in treatment effect over time Integrate Summaries of Safety and Efficacy Integrate existing information Provide data for rational decision making Prospective planning in drug development 9
- 10. CONTACT INFORMATION Primary Contact: President: Jian Chen (jian.chen@edetek.com) EDETEK, Inc. 1 Independence Way, Suite 405 Princeton, NJ 08540 Phone: 609-720-0888/9 Fax: 609-720-0880 E-mail: info@edetek.com 10