Electrochemistry
- 2. What is electrochemistry? ï Electrochemistry is the study of chemical reactions which take place at the interface of an electrode usually a solid, metal or semiconductor and an ionic conductor , the electrolyte. ï Electrochemistry deals with the interaction between electrical energy and chemical change.
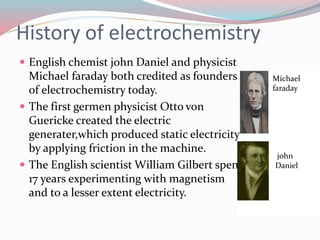
- 3. History of electrochemistry ï English chemist john Daniel and physicist Michael faraday both credited as founders of electrochemistry today. ï The first germen physicist Otto von Guericke created the electric generater,which produced static electricity by applying friction in the machine. ï The English scientist William Gilbert spent 17 years experimenting with magnetism and to a lesser extent electricity. Michael faraday john Daniel
- 4. ï The french chemist charles francois de cisternry du fay had discovered two types of static electricity. ï William Nicholson and Johann Wilhelm Ritter succeeded in decomposing water into hydrogen and oxygen by electrolysis. ï Ritter discovered the process of electroplating. ï William Hyde Wollaston made improvements to the galvanic cells. ï Orstedâs discovery of the magnetic effect of electrical currents and further work on electromagnetism to others.
- 5. ï Michael Faraday's experiments led him to state his two laws of electrochemistry and john Daniel invented primary cells. ï Paul Heroult and Charles M.Hall developed an efficient method to obtain aluminum using electrolysis of molten alumina. ï Nernst developed the theory of the electromotive force and his equation known as Nernst equation, which related the voltages of a cell to its properties. ï Quantum electrochemistry was developed by Revaz dogonadeze and his pupils.
- 6. Oxidation-Reduction ï The term redox stands for reduction-oxidation ï It refers to electrochemical processes involving electron transfer to or from a molecule or iron changing its states. ï The atom or molecule which loses electrons is known as the reducing agent. ï The substance which accepts the electrons is called the oxidizing agent.
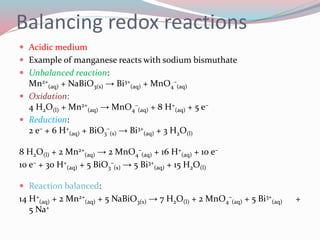
- 7. Balancing redox reactions ï Acidic medium ï Example of manganese reacts with sodium bismuthate ï Unbalanced reaction: Mn2+ (aq) + NaBiO3(s) â Bi3+ (aq) + MnO4 â (aq) ï Oxidation: 4 H2O(l) + Mn2+ (aq) â MnO4 â (aq) + 8 H+ (aq) + 5 eâ ï Reduction: 2 eâ + 6 H+ (aq) + BiO3 â (s) â Bi3+ (aq) + 3 H2O(l) 8 H2O(l) + 2 Mn2+ (aq) â 2 MnO4 â (aq) + 16 H+ (aq) + 10 eâ 10 eâ + 30 H+ (aq) + 5 BiO3 â (s) â 5 Bi3+ (aq) + 15 H2O(l) ï Reaction balanced: 14 H+ (aq) + 2 Mn2+ (aq) + 5 NaBiO3(s) â 7 H2O(l) + 2 MnO4 â (aq) + 5 Bi3+ (aq) + 5 Na+
- 8. ï Basic medium ï Example of reaction between potassium permanganate and sodium sulfite. ï Unbalanced reaction: KMnO4 + Na2SO3 + H2O â MnO2 + Na2SO4 + KOH ï Reduction: 3 eâ + 2 H2O + MnO4 â â MnO2 + 4 OHâ ï Oxidation: 2 OHâ + SO3 2â â SO4 2â + H2O + 2 eâ ï 6 eâ + 4 H2O + 2 MnO4 â â 2 MnO2 + 8 OHâ ï 6 OHâ + 3 SO3 2â â 3 SO4 2â + 3 H2O + 6eâ ï Equation balanced: 2 KMnO4 + 3 Na2SO3 + H2O â 2 MnO2 + 3 Na2SO4 + 2 KOH
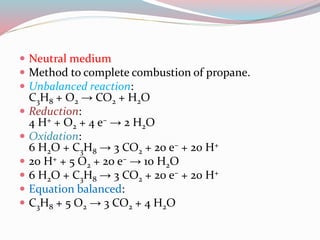
- 9. ï Neutral medium ï Method to complete combustion of propane. ï Unbalanced reaction: C3H8 + O2 â CO2 + H2O ï Reduction: 4 H+ + O2 + 4 eâ â 2 H2O ï Oxidation: 6 H2O + C3H8 â 3 CO2 + 20 eâ + 20 H+ ï 20 H+ + 5 O2 + 20 eâ â 10 H2O ï 6 H2O + C3H8 â 3 CO2 + 20 eâ + 20 H+ ï Equation balanced: ï C3H8 + 5 O2 â 3 CO2 + 4 H2O
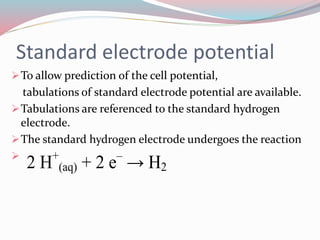
- 10. Standard electrode potential ïTo allow prediction of the cell potential, tabulations of standard electrode potential are available. ïTabulations are referenced to the standard hydrogen electrode. ïThe standard hydrogen electrode undergoes the reaction ï 2 H+ (aq) + 2 eâ â H2
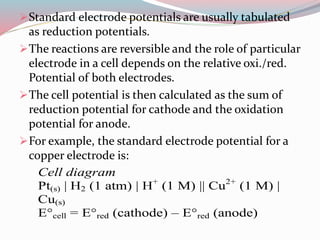
- 11. ïStandard electrode potentials are usually tabulated as reduction potentials. ïThe reactions are reversible and the role of particular electrode in a cell depends on the relative oxi./red. Potential of both electrodes. ïThe cell potential is then calculated as the sum of reduction potential for cathode and the oxidation potential for anode. ïFor example, the standard electrode potential for a copper electrode is: Cell diagram Pt(s) | H2 (1 atm) | H+ (1 M) || Cu2+ (1 M) | Cu(s) E°cell = E°red (cathode) â E°red (anode)
- 12. Gibbs free energy and cell potential ïThough cell potential Cell and get electricity n faraday in the cell: = -nFEcell For standard cell, this equation can we written 0= -RTlnK=-nFE0 G cell ïThough produce of electric energy converted into electric work, Wmax= Welectrical= -nFEcell
- 13. Nernst equation n+|M)=E0 (M E(M n+|M)- ln But solid M concentrate constant n+|M)=E0 (M E(M n+|M)- ln Example of Daniel cell 2+|Cu)=E0 (Cu For cathode : E(Cu 2+|Cu)- ln 2+|Zn)=E0 (Zn For anode : E(Zn 2+|Zn)- ln 2+|Cu) - E(Zn Cell Potential : Ecell= : E(Cu 2+|Zn) = E0 2+|Cu)- ln - E0 (Zn (Cu 2+|Zn)- ln = Ecell=E0 cell- ln
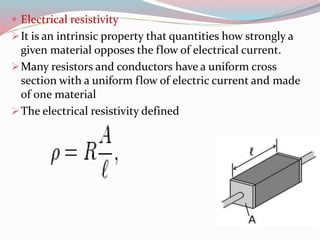
- 14. ï Electrical resistivity ïIt is an intrinsic property that quantities how strongly a given material opposes the flow of electrical current. ïMany resistors and conductors have a uniform cross section with a uniform flow of electric current and made of one material ïThe electrical resistivity defined
- 15. ï Electrical conductivity ïThe reciprocal of electrical resistivity, and measures a materialâs ability to conduct an electric current. ïIt is commonly represented by Ï ïConductivity is defined as Conductivity SI units of Siemens per meter.
- 16. Molar conductivity ïMolar conductivity is defined as the conductivity of an electrolyte solution divided by the molar concentration of the electrolyte, and so measures the efficiency with which a given electrolyte conducts electricity in solution. ïFrom definition, the molar conductivity
- 17. âĒ Two cases should be distinguished: ïStrong eletrolyte and weak electrolyte ï For strong electrolyte ïSalts, strong acids and strong bases, the molar conductivity depends only weakly on concentration.
- 18. ï For weak electrolyte ïThe molar conductivity strongly depends on concentration. ïThe more dilute a solution, the greater its molar conductivity, due to increased ionic dissociation. ïFor weak electrolyte obeys Oswald's dilulation law.
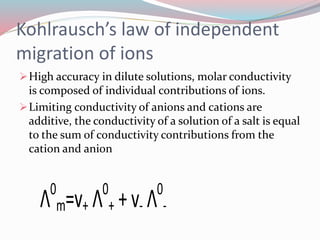
- 19. Kohlrauschâs law of independent migration of ions ïHigh accuracy in dilute solutions, molar conductivity is composed of individual contributions of ions. ïLimiting conductivity of anions and cations are additive, the conductivity of a solution of a salt is equal to the sum of conductivity contributions from the cation and anion Î0 m=v+ Î0 + + v- Î0 -
- 20. Battery ïMany types of battery have been commercialized and represent an important practical application of electrochemistry. ïEarly wet cells powered the first telegraph and telephone systems, and were the source of current for electroplating. ïThe zinc-manganese dioxide dry cell was the first portable, non-spill able battery type that made flashlights and other portable devices practical.
- 21. ïThe mercury battery using zinc and mercuric oxude provided higher levels of power and capacity than the original dry cell for early electronic devices. ïLead-acid battery was secondary battery. ïThe electrochemical reaction that produced current was reversible, allowing electrical energy and chemical energy to be interchanged as needed. ïLead-acid cells continue to be widely used in automobiles.
- 22. ïThe lithium battery, which does not use water in the electrolyte, provides improved performance over other types. ïRechargeable lithium ion battery is an essential part of many mobile devices.
- 23. Corrosion ïCorrosion is the term applied to steel rust caused by an electrochemical process. ïCorrosion of iron in the form of reddish rust, black tarnish on silver, red or green may be appear on copper and its alloys, such as brass.
- 24. Prevention of corrosion ï Coating ïMetals can be coated with paint or other less conductive metals. ïThis prevents the metal surface from being exposed to electrolytes. ïScratches exposing the metal substrate will result in corrosion.
- 25. âĒ Sacrificial anodes ïThe method commonly used to protect a structural metal is to attach a metal which is more anodic than the metal to be protected. ïThis forces the structural metal to be catholic thus spared corrosion. it is called sacrificial. ïZinc bars are attached to various locations on steel ship hulls to render the ship hull catholic. ïOther metal used magnesium.
- 26. Electrolysis ïThe spontaneous redox reactions of a conventional battery produce electricity through the different chemical potentials of the cathode and anode in the electrolyte. ïElectrolysis requires an external source of electrical energy to include a chemical reaction , and this process takes place in a compartment called an electrolytic cell.
- 27. Electrolysis of molten sodium chlorine ïWhen molten, the salt sodium chloride can be electrolyzed to yield metallic sodium and gaseous chlorine. ïThis process takes place in a special cell named Downâs cell. Reactions that take place at Down's cell are the following Anode (oxidation): 2 Clâ â Cl2(g) + 2 eâ Cathode (reduction): 2 Na+ (l) + 2 eâ â 2 Na(l) Overall reaction: 2 Na+ + 2 Clâ (l) â 2 Na(l) + Cl2(g) ïThis process can yield large amounts of metallic sodium and gaseous chlorine, and widely used on mineral dressing and metallurgy industries.
- 28. Quantitative electrolysis and Faradayâs law ïQuantitative aspects of electrolysis were originally developed by Michel faraday . ïFaraday is also credited to have coined the terms electrolyte. ïElectrolysis among many others while studying analysis of electrochemical reactions. ïFaraday advocate of the law of conservation of energy.
- 29. First law ï The mass of products yielded on the electrodes was proportional to the the value of current supplied to the cell, the length of time the current existed, and the molar mass of the substance analyzed. ï The amount of substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity passed through the cell. m=
- 30. Second law ïThe amounts of bodies which are equivalent to each other in the ordinary chemical action have equal quantities of of electricity naturally associated with them. ïThe quantities of different elements deposited by a given amount of electricity are in the ratio of the chemical equivalent weights
- 31. Applied aspects of electrochemistry ïIndustrial electrolytic processes ïElectrochemical Reactors ïBatteries ïFuel cells ïSome Electrochemical Devices ïElectrochemical Methods of Analysis
- 32. Branch of electrochemistry ï Photo electrochemistry ïIt is subfield of study within physical chemistry. ïThe interest in this domain is high in the context of development of renewable energy conversion and storage technology. ïThe effects of luminous radiation on the properties of electrodes and on electrochemical reactions are the subject of photo electrochemistry
- 33. ï Semiconductorâs electrochemistry ïSemiconductor material has a band gap and generates a pair of electron and hole per absorbed photon if the energy of the photon is higher than the band gap of the semiconductor. ïThis property of semiconductor materials has been successfully used to converted solar energy into electrical energy by photovoltaic devices. ï Semiconductor-electrolyte interface ïWhen a semiconductor comes into contact with a liquid, to maintain electrostatic equillibrium ïThere will be a charge transfer between the semiconductor and liquid phase,if formal redox potential of redox species lies inside semiconductor band gap.
- 34. ïAt thermodynamic eqilibrium, the fermi level of semiconductor and the formal redox potential of redox species and between interface semiconductor. ïThis introduce n-type semiconductor and p-type semiconductor. ïThis semiconductor used as photovoltaic device similar to solid state p-n junction devices. ïBoth n and p type semiconductor can used as photovoltaic devices to convert solar energy into electrical energy and are called photoelectrical cells
- 35. ï Boielectrochemistry ïIt is branch of electrochemistry and biophysical chemistry concerned with topics like cell electron-proton transport, cell membrane potentials and electrode reactions of redo enzymes. ïBioelectrochemistry is a science at the many junctions of sciences.
- 36. Nanoelectrochemistry ïNanoelectrochemistry is a branch of electrochemistry that investigates the electrical and electrochemical properties of materials at the nanometer size regime. ïNanoelectrochemistry plays significant role in the fabrication of various sensors, and devices for detecting molecules at very law concentrations.
- 37. ïThe term electrochemical nanostructuring can be used to mean different things. ïThis term is employed to refer to generation at will of nanostructure on electrode surface, involving a given positioning with a certain precision ïThe term nanostructure is used to describe the generation of nanometric patterns with move or less narrow size distribution and a periodic or random ordering on the surface. ïBut without control on the spatial location of the nanostructure.
- 38. Application of electrochemistry ïThere are various extremely important electrochemical processes in both nature and industry. ïThe coating of objects with metals or metal oxides through electro deposition and the detection of alcohol in drunken drivers through the redox reaction of ethanol. ïDiabetes blood sugar meters measure the amount of glucose in the blood through its redox potential.
- 39. ïThe generation of chemical energy through photosynthesis in inherently an electrochemical process. ïProduction of metals like aluminium and titanium from their ores. ï For Photo electrochemistry ïArtificial photosynthesis ïRegenerative cell or Dye-sensitized cell ïPhoto electrochemical splitting of water
- 40. ï For Boielectrochemistry ïSome of different experimental techniques that can be used to study bioelectrochemical problems. ïAmpermetic of biosensors ïBiofuel cells ïBioelectrosynthesis