Electronic Submissions
- 1. US FDA Regulation UpdateElectronic SubmissionsPresented by John Beasley
- 2. TopicsElectronic SubmissionsFDA Electronic Submission Gateway (ESG)CDRH initiativeseSubmitter / WebTrader SoftwareVideo
- 3. Introduction
- 5. IntroductionDrugs (Regulated by CDER) - Over-the-Counter medications- Prescription Drugs- Generic DrugsBiologics (Regulated by CBER) - Vaccines- Blood- Gene TherapiesFood & Cosmetics (Regulated by CFSAN)- Food products (excluding meat)- Food additives (colors, fortifications, radiation)- Dietary Supplements- Bottled Water- Food packaging and labeling- CosmeticsMedical Devices (Regulated by CDRH) - Wheelchairs- Bandages- Contact Lenses- Prosthetic Limbs- MRI MachinesRadiation-Emitting Products (Regulated by CDRH) - Microwave Ovens- Televisions- Medical X-ray machines- Baggage X-ray machinesVeterinary Products (Regulated by CVM) - Livestock Feed- Pet Food- Animal Drugs
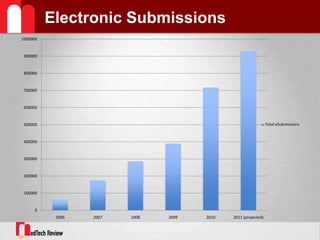
- 6. Electronic Submissions1997 ¨C Computer Assisted New Drug Applications2001 ¨C Providing Regulatory eSubmissions for NDAs, BLAs, ANDAs2007 ¨C CDRH e-copy initiatives; turbo 510(k) for IVD products2011 ¨C eMDR (21 CFR 803)
- 7. Electronic SubmissionsSTDM ¨C statistical time division multiplexingHL7 ¨C standards for interoperability of health information technology
- 8. Electronic SubmissionsFDA Electronic Submission GatewayFederal Register: August 8, 2006 (Volume 71, Number 152)A single point of entry for receiving and processing all electronic submissions in a highly secure environment,Automating current processes such as the electronic acknowledgment of submissions, andSupporting the electronic Common Technical Document (eCTD).
- 11. Electronic Submission21 CFR 33321 CFR 880.6890
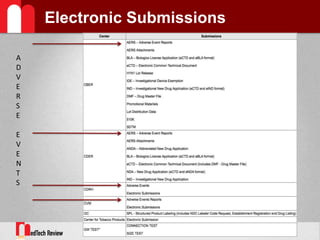
- 12. Electronic Submissions GatewayReceiptmeans transfer of a submission from a senderˇŻs system to a temporary storage area in the GATEWAY (FDA ESG).Acknowledgmentto the sender that the submission was sent from the senderˇŻs system and received by the Gateway.Routingrefers to delivering a submission to a Center-level storage area and initiating a load process to place a submission into a Center receiving systemNotificationof a submissionˇŻs arrival is made to those individuals responsible for the CenterˇŻs receiving system.
- 13. Electronic Submissions GatewayThe Gateway is a conduit, or "highway", along which submissions travel to reach their final destination.
- 14. Electronic Submissions Gateway Six to eight weeks to setup
- 15. Less expensive
- 16. Greater accuracy
- 17. Fast
- 18. Better evidenceWhatˇŻs in it for you?CollaborationCost reductionRecord retentionResponsivenessSingle environment for all AE
- 20. FDAˇŻs Electronic Submission GatewayLetter of non-repudiationDUNS numberDigital certificatePrepare electronic submissionSet up the computerLetter of Non-repudiationAS2 Gateway to GatewayESGDigital certificateeSubmitter & WebTrader
- 21. US FDA Webinar ¨C June 2011