Elements and compounds (1) (1)
ŌĆóDownload as PPTX, PDFŌĆó
1 likeŌĆó183 views
elements and compounds and there differences . The periodic table with the creator and a small trivia to enjoy.!!!
1 of 19
Download to read offline

















Recommended
Chapter 1 - Introduction to Chemistry



Chapter 1 - Introduction to ChemistryKendon Smith
╠²
This presentation covers Chapter 1 and the Introduction to Chemistry, including the Scientific Method.Atoms, molecules, elements, compounds, mixtures and solutions



Atoms, molecules, elements, compounds, mixtures and solutionssafa-medaney
╠²
The document defines key chemistry terms including atoms, molecules, elements, compounds, mixtures and solutions. Atoms are the smallest unit of matter that cannot be divided further. Molecules are formed when two or more atoms combine chemically. Matter is made up of elements, compounds, mixtures and solutions. Elements are made of the same type of atom, compounds contain two or more types of atoms bonded together with a specific chemical formula. Mixtures contain substances that are not chemically combined, while solutions occur when a substance dissolves evenly in another.Atoms, elements, compounds and mixtures.pptx



Atoms, elements, compounds and mixtures.pptxSoniaTaneja15
╠²
1) The document discusses atoms, elements, compounds, and mixtures. It aims to explain what an atom is, differentiate between elements, compounds and mixtures, and give examples of each.
2) Atoms are the basic building blocks of all matter and are very small. Elements are substances made of only one type of atom that cannot be broken down further.
3) Compounds are formed when two or more elements are chemically bonded together and have different properties than the original elements. Mixtures contain two or more substances that are not chemically bonded and can be separated.The mole concept



The mole conceptmae2388
╠²
This document discusses moles, molar mass, and Avogadro's number. It explains that a mole is the amount of a substance that contains 6.022x1023 particles, known as Avogadro's number. It also defines molar mass as the mass in grams of one mole of a substance. The document provides examples of calculating molar mass from atomic masses and using molar mass to determine the number of moles or particles in a given mass of a substance.Periodicity



PeriodicityHoshi94
╠²
1. The document discusses periodicity and trends in the properties of elements as they relate to their position in the periodic table.
2. Key periodic trends include decreases in atomic radius and increases in ionization energy and electronegativity from left to right across a period, and the opposite trends down a group.
3. The placement of elements in the periodic table allows for predictions of element properties and reactivity based on electronic structure.Atomic number, Mass number, Relative atomic mass and Atomic mass unit



Atomic number, Mass number, Relative atomic mass and Atomic mass unitQazi GHAFOOR
╠²
The document discusses atomic number, mass number, and relative atomic mass. It states that the atomic number of an element equals the number of protons in its nucleus. The mass number is the sum of protons and neutrons. An example problem finds an atom has 92 protons and 146 neutrons from a given mass number of 238 and atomic number of 92. Relative atomic mass is the average mass of an atom's isotope compared to carbon-12, measured in atomic mass units.Chemical bonding



Chemical bondingPepi Jaramillo Romero
╠²
The document outlines a lesson plan on chemical bonding. It will cover three main topics: 1) an introduction to chemical bonds, 2) the different types of chemical bonds including ionic, covalent and metallic bonds, and 3) the nomenclature of inorganic chemistry according to IUPAC recommendations. The lesson aims to explain how atoms bond to form molecules or compounds through electron sharing or transfer. It will also describe the various bond types and properties that distinguish ionic, covalent and metallic substances. Activities are included to reinforce key concepts.Introduction To Chemistry



Introduction To ChemistryOH TEIK BIN
╠²
A Power Point Presentation on Introductory Chemistry. To motivate new students of Chemistry. To help students appreciate the importance of Chemicals in everyday life. Done by Bro. Oh Teik Bin, Lower Perak Buddhist Association, Teluk Intan, Malaysia.Elements and Compounds



Elements and CompoundsJeremy Mularella
╠²
- Elements are pure substances that cannot be broken down further without losing their identity, and there are currently 118 known elements, with 88 occurring naturally.
- In the universe, hydrogen makes up 75% and helium 20%, while on Earth oxygen is the most abundant element in the crust at 46.6% and silicon is second most at 27.7%.
- Compounds are pure substances made of two or more chemically bonded elements, with properties different from the individual elements, and can be represented by chemical formulas showing the elements and their ratios.26. covalent bonding



26. covalent bondingFatih G├Č├¦mez
╠²
Atoms form bonds to achieve stable electron configurations. Covalent bonds form when atoms share valence electrons to fill their outer shells. Different bonding structures lead to varied properties. Diamond has a giant covalent structure where each carbon atom bonds to four others in a 3D network, giving it properties like hardness. Graphite also contains carbon but its layers can slide due to weaker bonds between layers, making it soft.Lesson 3 Properties of Liquid.pptx



Lesson 3 Properties of Liquid.pptxelainejanecarreon
╠²
This document discusses the properties of liquid and water. It explains that liquids have more space between particles than solids, allowing particles to flow and change shape while maintaining a constant volume. The strongest intermolecular force in water is hydrogen bonding between molecules. Stronger intermolecular forces lead to higher boiling points, viscosity, surface tension and heat of vaporization but lower vapor pressure. Properties like the polarity and hydrogen bonding of water molecules allow it to have unique properties like being an excellent solvent.HISTORY OF ATOMIC THEORY



HISTORY OF ATOMIC THEORYJimnaira Abanto
╠²
John Dalton proposed the atomic theory in 1804, stating that all matter is composed of tiny, indivisible particles called atoms that cannot be divided further. Later discoveries found that atoms consist of even smaller subatomic particles, including electrons discovered by J.J. Thomson in 1897 and the nucleus discovered by Ernest Rutherford in 1910. The quantum mechanical model developed in 1926 by Schrodinger, Heisenberg and others proposed that electrons exist as waves of energy around the nucleus, rather than following fixed orbits as proposed by Niels Bohr's 1913 planetary model of the atom.8 e atoms & elements (boardworks)



8 e atoms & elements (boardworks)cartlidge
╠²
This document is about atoms, elements, and chemical reactions. It contains three main sections: (1) what atoms and elements are, including their symbols and arrangement in the periodic table, (2) how elements can combine to form compounds with different properties, and (3) how chemical reactions allow atoms to join together to form new substances.Molecular Compounds



Molecular CompoundsOhMiss
╠²
Molecular compounds are formed when atoms of two or more different non-metals combine through covalent bonds. Covalent bonds involve the sharing of electron pairs between atoms. Molecular compounds have properties such as being soft, having low melting points, and not conducting electricity. The naming of molecular compounds involves naming the elements present and indicating the number of atoms through prefixes.Atomic Structure and the Periodic Table



Atomic Structure and the Periodic TablePaul Schumann
╠²
This document provides an overview of the history and development of atomic theory, including key discoveries and models. It describes early ideas from Democritus and Aristotle, foundations laid by laws of conservation of mass, definite proportions, and multiple proportions. Developments include Dalton's atomic theory, discovery of the electron, proton, neutron, isotopes, and the nuclear model of the atom. The periodic table is introduced along with atomic number, mass number, and calculating average atomic mass.How to write a chemical formula



How to write a chemical formulaZahraFazal6
╠²
This document discusses how to write chemical formulas. It explains that a chemical formula tells us the types and numbers of atoms in a compound. It then outlines five steps for writing chemical formulas: 1) Write the symbols of the ions side by side in order of positive then negative, 2) Write the valency of each ion, 3) Transfer the valencies to offset them, 4) Omit valencies that are the same, and include different valencies in the formula, 5) For radicals like sulfate and phosphate, the net charge indicates the radical's valency and it is written in parentheses.Chemistry...What, Why, How



Chemistry...What, Why, HowOH TEIK BIN
╠²
This document provides an introduction to chemistry. It defines chemistry as the scientific study of substances, their structures, properties, and reactions. It explains that chemistry is important for many career fields like medicine, engineering, and agriculture. It also discusses how we can study chemistry effectively through reading, relating concepts to everyday life, solving problems, using visualizations and organizing notes. Finally, it outlines the format of chemistry exams for SPM and STPM levels in Malaysia.Introduction to chemistry



Introduction to chemistryRuba Salah
╠²
The document discusses the key differences between elements, compounds, and mixtures. It defines an element as a pure substance made of only one type of atom. A compound is a pure substance composed of two or more elements that are chemically combined, forming molecules. A mixture is a combination of two or more substances that are not chemically combined and can be separated through physical means alone.Structure of atom (igcse)



Structure of atom (igcse)MOHAMMED AHSAN
╠²
1. Atoms consist of a positively charged nucleus surrounded by electrons that orbit in defined shells or energy levels.
2. The number of protons in the nucleus defines the atomic number of an element, while the total number of protons and neutrons gives the mass number.
3. Chemical properties are determined by valence electrons in the outer shell. Elements tend to gain or lose electrons to achieve a stable outer shell of 8 electrons.MOLE; Avogadro's Number; Percentage Composition



MOLE; Avogadro's Number; Percentage CompositionJimnaira Abanto
╠²
The document defines key chemistry concepts related to moles, including:
- A mole refers to Avogadro's number (6.02x10^23) of particles like atoms, molecules, ions or formula units.
- 1 mole of an element contains 6.02x10^23 atoms, 1 mole of a molecular compound contains 6.02x10^23 molecules, and 1 mole of an ionic compound contains 6.02x10^23 formula units.
- Gram atomic mass refers to the mass of one mole of an element in grams, and gram formula mass refers to the sum of atomic weights that make up one mole of a compound.
- Chemical formulas represent theCOMPOUNDS



COMPOUNDSDavid Young
╠²
A compound is a pure substance composed of two or more elements chemically bonded together, such as sodium chloride or water. Compounds can only be broken down or formed through a chemical reaction that breaks and rearranges bonds between atoms. Some common compounds found in homes include sodium chloride, water, glucose, and other substances containing ionically or covalently bonded elements.atoms and molecules



atoms and moleculesGaurav Vashisht
╠²
This document is a chemistry student's report on atoms and molecules. It begins with an introduction discussing the molecular structure hypothesis and how it relates to quantum mechanics. It then covers elemental symbols, compound formulas, the structure of atoms including protons, neutrons, electrons and isotopes. Finally, it discusses atomic mass units, atomic weights, molecular weights, and how the mole concept applies to elements and compounds for chemical calculations. The key topics are represented in less than 3 sentences.Ionic Bonding



Ionic BondingCurrituck County High School
╠²
This document discusses ionic and metallic bonding. It explains that ions are formed when atoms gain or lose electrons to achieve stable noble gas electron configurations. Metals form cations by losing electrons while nonmetals form anions by gaining electrons. Ionic compounds contain cations and anions in ratios represented by chemical formulas. Metallic bonding occurs via delocalized valence electrons that are shared between metal atoms.PURE SUBSTANCES 



PURE SUBSTANCES Gaurav Arora
╠²
This document defines pure substances and mixtures. A pure substance is homogeneous and has definite properties, while a mixture contains two or more substances mixed together without chemical change. Solutions are homogeneous mixtures where particle size is molecular. Suspensions are heterogeneous mixtures where particle size is larger, allowing settling. Colloids have intermediate particle sizes that do not settle. The document discusses types of mixtures and their distinguishing characteristics.Chemical formula ppt



Chemical formula pptShree Kutty
╠²
Valency refers to an element's combining power, or how its atoms will bond with atoms of other elements to form compounds. There are rules for writing chemical formulas: the symbols of elements and their valencies are written first, metals are written before non-metals to form compounds, and polyatomic ions are enclosed in brackets with their ratios. Valencies must be crossed off as atoms combine in a chemical formula.Biology base and acid



Biology base and acidM, Michelle Jeannite
╠²
This document discusses acids and bases, including their properties and how to test for them. It provides information on:
- The pH scale ranges from 0-14, with acids having lower values and bases having higher values. A pH of 7 is neutral.
- Acids donate H+ ions in solution and have a pH below 7. Bases donate OH- ions and have a pH above 7.
- Indicators like litmus paper and cabbage juice can be used to test if a substance is acidic or basic - acids turn indicators one color while bases turn them another.
- Common household substances like lemon juice, vinegar, baking soda and bleach are identified along the pH scale.
- AClassifications of Matter



Classifications of MatterSimple ABbieC
╠²
This document defines the classification of matter. There are two main categories: pure substances and mixtures. Pure substances include elements, which are made of only one type of atom, and compounds, which are two or more elements chemically bonded together. Mixtures contain two or more pure substances mixed together without chemical bonding. Mixtures can be either heterogeneous, where the parts can be seen, or homogeneous, where the parts cannot be seen. Heterogeneous mixtures are less pure than homogeneous mixtures.Chapter 3



Chapter 3Lama K Banna
╠²
First Year in Dentistry First Semester
Al-Azhar University - Gaza
Lama El Banna
general chemistry Elements, compound and mixture



Elements, compound and mixturebrintha smith
╠²
This document discusses the classification and properties of matter. It defines the four states of matter as solid, liquid, gas, and plasma. Matter is classified as either elements, compounds, or mixtures based on its chemical constitution. Elements are pure substances that cannot be broken down further, while compounds contain two or more elements chemically bonded together. Compounds have distinct properties from their constituent elements. The document provides examples of elements and compounds, and discusses their distinguishing physical and chemical properties. Redox reactions are described as reactions where both oxidation and reduction occur simultaneously.Ch.10 L 1 using the periodic table.pptx



Ch.10 L 1 using the periodic table.pptxAmlHanafi
╠²
The document traces the development of the periodic table from early lists of elements compiled by scientists like Lavoisier to Mendeleev's groundbreaking periodic table that included predictive properties. It organized elements by atomic mass and left gaps for undiscovered elements, correctly predicting properties of three. Moseley later reorganized the table by atomic number, establishing the modern periodic table's clear periodic trends when arranged by this property. The document also outlines key properties of metals, nonmetals, and metalloids and how they are grouped on the periodic table.More Related Content
What's hot (20)
Elements and Compounds



Elements and CompoundsJeremy Mularella
╠²
- Elements are pure substances that cannot be broken down further without losing their identity, and there are currently 118 known elements, with 88 occurring naturally.
- In the universe, hydrogen makes up 75% and helium 20%, while on Earth oxygen is the most abundant element in the crust at 46.6% and silicon is second most at 27.7%.
- Compounds are pure substances made of two or more chemically bonded elements, with properties different from the individual elements, and can be represented by chemical formulas showing the elements and their ratios.26. covalent bonding



26. covalent bondingFatih G├Č├¦mez
╠²
Atoms form bonds to achieve stable electron configurations. Covalent bonds form when atoms share valence electrons to fill their outer shells. Different bonding structures lead to varied properties. Diamond has a giant covalent structure where each carbon atom bonds to four others in a 3D network, giving it properties like hardness. Graphite also contains carbon but its layers can slide due to weaker bonds between layers, making it soft.Lesson 3 Properties of Liquid.pptx



Lesson 3 Properties of Liquid.pptxelainejanecarreon
╠²
This document discusses the properties of liquid and water. It explains that liquids have more space between particles than solids, allowing particles to flow and change shape while maintaining a constant volume. The strongest intermolecular force in water is hydrogen bonding between molecules. Stronger intermolecular forces lead to higher boiling points, viscosity, surface tension and heat of vaporization but lower vapor pressure. Properties like the polarity and hydrogen bonding of water molecules allow it to have unique properties like being an excellent solvent.HISTORY OF ATOMIC THEORY



HISTORY OF ATOMIC THEORYJimnaira Abanto
╠²
John Dalton proposed the atomic theory in 1804, stating that all matter is composed of tiny, indivisible particles called atoms that cannot be divided further. Later discoveries found that atoms consist of even smaller subatomic particles, including electrons discovered by J.J. Thomson in 1897 and the nucleus discovered by Ernest Rutherford in 1910. The quantum mechanical model developed in 1926 by Schrodinger, Heisenberg and others proposed that electrons exist as waves of energy around the nucleus, rather than following fixed orbits as proposed by Niels Bohr's 1913 planetary model of the atom.8 e atoms & elements (boardworks)



8 e atoms & elements (boardworks)cartlidge
╠²
This document is about atoms, elements, and chemical reactions. It contains three main sections: (1) what atoms and elements are, including their symbols and arrangement in the periodic table, (2) how elements can combine to form compounds with different properties, and (3) how chemical reactions allow atoms to join together to form new substances.Molecular Compounds



Molecular CompoundsOhMiss
╠²
Molecular compounds are formed when atoms of two or more different non-metals combine through covalent bonds. Covalent bonds involve the sharing of electron pairs between atoms. Molecular compounds have properties such as being soft, having low melting points, and not conducting electricity. The naming of molecular compounds involves naming the elements present and indicating the number of atoms through prefixes.Atomic Structure and the Periodic Table



Atomic Structure and the Periodic TablePaul Schumann
╠²
This document provides an overview of the history and development of atomic theory, including key discoveries and models. It describes early ideas from Democritus and Aristotle, foundations laid by laws of conservation of mass, definite proportions, and multiple proportions. Developments include Dalton's atomic theory, discovery of the electron, proton, neutron, isotopes, and the nuclear model of the atom. The periodic table is introduced along with atomic number, mass number, and calculating average atomic mass.How to write a chemical formula



How to write a chemical formulaZahraFazal6
╠²
This document discusses how to write chemical formulas. It explains that a chemical formula tells us the types and numbers of atoms in a compound. It then outlines five steps for writing chemical formulas: 1) Write the symbols of the ions side by side in order of positive then negative, 2) Write the valency of each ion, 3) Transfer the valencies to offset them, 4) Omit valencies that are the same, and include different valencies in the formula, 5) For radicals like sulfate and phosphate, the net charge indicates the radical's valency and it is written in parentheses.Chemistry...What, Why, How



Chemistry...What, Why, HowOH TEIK BIN
╠²
This document provides an introduction to chemistry. It defines chemistry as the scientific study of substances, their structures, properties, and reactions. It explains that chemistry is important for many career fields like medicine, engineering, and agriculture. It also discusses how we can study chemistry effectively through reading, relating concepts to everyday life, solving problems, using visualizations and organizing notes. Finally, it outlines the format of chemistry exams for SPM and STPM levels in Malaysia.Introduction to chemistry



Introduction to chemistryRuba Salah
╠²
The document discusses the key differences between elements, compounds, and mixtures. It defines an element as a pure substance made of only one type of atom. A compound is a pure substance composed of two or more elements that are chemically combined, forming molecules. A mixture is a combination of two or more substances that are not chemically combined and can be separated through physical means alone.Structure of atom (igcse)



Structure of atom (igcse)MOHAMMED AHSAN
╠²
1. Atoms consist of a positively charged nucleus surrounded by electrons that orbit in defined shells or energy levels.
2. The number of protons in the nucleus defines the atomic number of an element, while the total number of protons and neutrons gives the mass number.
3. Chemical properties are determined by valence electrons in the outer shell. Elements tend to gain or lose electrons to achieve a stable outer shell of 8 electrons.MOLE; Avogadro's Number; Percentage Composition



MOLE; Avogadro's Number; Percentage CompositionJimnaira Abanto
╠²
The document defines key chemistry concepts related to moles, including:
- A mole refers to Avogadro's number (6.02x10^23) of particles like atoms, molecules, ions or formula units.
- 1 mole of an element contains 6.02x10^23 atoms, 1 mole of a molecular compound contains 6.02x10^23 molecules, and 1 mole of an ionic compound contains 6.02x10^23 formula units.
- Gram atomic mass refers to the mass of one mole of an element in grams, and gram formula mass refers to the sum of atomic weights that make up one mole of a compound.
- Chemical formulas represent theCOMPOUNDS



COMPOUNDSDavid Young
╠²
A compound is a pure substance composed of two or more elements chemically bonded together, such as sodium chloride or water. Compounds can only be broken down or formed through a chemical reaction that breaks and rearranges bonds between atoms. Some common compounds found in homes include sodium chloride, water, glucose, and other substances containing ionically or covalently bonded elements.atoms and molecules



atoms and moleculesGaurav Vashisht
╠²
This document is a chemistry student's report on atoms and molecules. It begins with an introduction discussing the molecular structure hypothesis and how it relates to quantum mechanics. It then covers elemental symbols, compound formulas, the structure of atoms including protons, neutrons, electrons and isotopes. Finally, it discusses atomic mass units, atomic weights, molecular weights, and how the mole concept applies to elements and compounds for chemical calculations. The key topics are represented in less than 3 sentences.Ionic Bonding



Ionic BondingCurrituck County High School
╠²
This document discusses ionic and metallic bonding. It explains that ions are formed when atoms gain or lose electrons to achieve stable noble gas electron configurations. Metals form cations by losing electrons while nonmetals form anions by gaining electrons. Ionic compounds contain cations and anions in ratios represented by chemical formulas. Metallic bonding occurs via delocalized valence electrons that are shared between metal atoms.PURE SUBSTANCES 



PURE SUBSTANCES Gaurav Arora
╠²
This document defines pure substances and mixtures. A pure substance is homogeneous and has definite properties, while a mixture contains two or more substances mixed together without chemical change. Solutions are homogeneous mixtures where particle size is molecular. Suspensions are heterogeneous mixtures where particle size is larger, allowing settling. Colloids have intermediate particle sizes that do not settle. The document discusses types of mixtures and their distinguishing characteristics.Chemical formula ppt



Chemical formula pptShree Kutty
╠²
Valency refers to an element's combining power, or how its atoms will bond with atoms of other elements to form compounds. There are rules for writing chemical formulas: the symbols of elements and their valencies are written first, metals are written before non-metals to form compounds, and polyatomic ions are enclosed in brackets with their ratios. Valencies must be crossed off as atoms combine in a chemical formula.Biology base and acid



Biology base and acidM, Michelle Jeannite
╠²
This document discusses acids and bases, including their properties and how to test for them. It provides information on:
- The pH scale ranges from 0-14, with acids having lower values and bases having higher values. A pH of 7 is neutral.
- Acids donate H+ ions in solution and have a pH below 7. Bases donate OH- ions and have a pH above 7.
- Indicators like litmus paper and cabbage juice can be used to test if a substance is acidic or basic - acids turn indicators one color while bases turn them another.
- Common household substances like lemon juice, vinegar, baking soda and bleach are identified along the pH scale.
- AClassifications of Matter



Classifications of MatterSimple ABbieC
╠²
This document defines the classification of matter. There are two main categories: pure substances and mixtures. Pure substances include elements, which are made of only one type of atom, and compounds, which are two or more elements chemically bonded together. Mixtures contain two or more pure substances mixed together without chemical bonding. Mixtures can be either heterogeneous, where the parts can be seen, or homogeneous, where the parts cannot be seen. Heterogeneous mixtures are less pure than homogeneous mixtures.Chapter 3



Chapter 3Lama K Banna
╠²
First Year in Dentistry First Semester
Al-Azhar University - Gaza
Lama El Banna
general chemistry Similar to Elements and compounds (1) (1) (20)
Elements, compound and mixture



Elements, compound and mixturebrintha smith
╠²
This document discusses the classification and properties of matter. It defines the four states of matter as solid, liquid, gas, and plasma. Matter is classified as either elements, compounds, or mixtures based on its chemical constitution. Elements are pure substances that cannot be broken down further, while compounds contain two or more elements chemically bonded together. Compounds have distinct properties from their constituent elements. The document provides examples of elements and compounds, and discusses their distinguishing physical and chemical properties. Redox reactions are described as reactions where both oxidation and reduction occur simultaneously.Ch.10 L 1 using the periodic table.pptx



Ch.10 L 1 using the periodic table.pptxAmlHanafi
╠²
The document traces the development of the periodic table from early lists of elements compiled by scientists like Lavoisier to Mendeleev's groundbreaking periodic table that included predictive properties. It organized elements by atomic mass and left gaps for undiscovered elements, correctly predicting properties of three. Moseley later reorganized the table by atomic number, establishing the modern periodic table's clear periodic trends when arranged by this property. The document also outlines key properties of metals, nonmetals, and metalloids and how they are grouped on the periodic table.Elements and the periodic table



Elements and the periodic tablemsali_aphs
╠²
The periodic table organizes the chemical elements and provides information about their properties and reactions. Elements are arranged in order of increasing atomic number and are grouped together based on similar chemical properties. The position of an element in the periodic table can reveal whether it is a metal, nonmetal, or noble gas, as well as provide details about its reactivity and physical state. Element symbols represent the elements concisely and are typically derived from each element's name.Elements and compounds



Elements and compoundsNolwazi Mabuza
╠²
This document provides an overview of elements, compounds, and how they relate. It defines elements as pure substances made of single atom types, while compounds are formed when two or more different elements bond together. Elements are represented by symbols on the periodic table and have distinct properties based on their atomic structure. Compounds have new properties and are represented by formulas showing the elements present and their ratios. The document explains how ionic and covalent bonds form compounds from elements and provides examples of common elements, compounds, and how to determine compound formulas from their constituent ions.Ch8 the atom-part 3



Ch8 the atom-part 3Beulah Heights University
╠²
The document provides an overview of the periodic table and classification of elements and matter. It discusses how elements are classified based on their properties, including metals and nonmetals. Key periodic patterns are described, such as how the chemical behavior of elements is determined by their electron configuration. The periodic law is explained, as well as the development of the modern periodic table with periods and families.Atoms and atomic theory review



Atoms and atomic theory reviewK Lipinski
╠²
This document provides information about classifying matter and its composition. It defines pure substances as elements or compounds made of uniform particles and mixtures as substances with two or more types of particles. Pure substances undergo physical or chemical changes, which respectively involve changes in properties or the formation of new substances. The document also discusses atoms as the basic building blocks of matter, containing subatomic particles like protons, neutrons, and electrons. It introduces the periodic table as organizing the elements by their chemical properties and number of protons.1.1 periodic table



1.1 periodic tableMartin Brown
╠²
1. The document summarizes the history and development of the periodic table, including contributions from Greek philosophers, Boyle, Davy, Moseley, Dobereiner, Newlands, and Mendeleev.
2. It describes the key features and organization of the modern periodic table, including periods, groups, atomic number, valence electrons, and trends in physical/chemical properties for different groups like alkali metals, alkaline earth metals, halogens, noble gases, and transition metals.
3. Specific elements are highlighted from different groups to illustrate trends, including lithium, sodium, potassium, beryllium, barium, calcium, magnesium, strontium, radium, chlorine, bromPPT in Science 7 Week 2.pptx



PPT in Science 7 Week 2.pptxJoycePerez27
╠²
This document defines elements, compounds, and mixtures. It provides examples of common elements like hydrogen, oxygen, and carbon. Elements are made of atoms and cannot be broken down further. Compounds are formed when elements chemically combine in molecules. Compounds have different properties than their constituent elements. Mixtures contain elements or compounds mixed together but not chemically combined. Mixtures can be either homogeneous, with a uniform composition, or heterogeneous, containing distinct substances or phases.PPT in Science 7 Week 2.pptx



PPT in Science 7 Week 2.pptxJoycePerez27
╠²
This document defines elements, compounds, and mixtures. It provides examples of common elements like hydrogen, oxygen, and carbon. Elements are made of atoms and cannot be broken down further. Compounds are formed when elements chemically bond together to form molecules. Compounds have distinct properties from their constituent elements. Mixtures contain elements or compounds mixed together but not chemically bonded. Mixtures can be either homogeneous, with a uniform composition, or heterogeneous, containing distinct substances that can be seen separately.Learning_The_Periodic_Table.ppt



Learning_The_Periodic_Table.pptMuhammadSamy18
╠²
Here are some common elements we use every day:
- Carbon - found in pencils, food, plastics
- Oxygen - we breathe it
- Hydrogen - found in water
- Nitrogen - in the air we breathe
- Calcium - in our bones and teeth
- Sodium - in salt
- Chlorine - in drinking water and swimming pools
- Aluminum - used in cans and foil
- Copper - in wiring and plumbing
- Iron - in foods and construction materials
- Zinc - in galvanization and supplements
- Iodine - added to salt for thyroid healthLearning_The_Periodic_Table.pptggggggggggggggggg



Learning_The_Periodic_Table.pptgggggggggggggggggredcarbin
╠²
Here are some common elements we use every day:
- Carbon - found in pencils, food, plastics
- Oxygen - we breathe it
- Hydrogen - found in water
- Nitrogen - in the air we breathe
- Calcium - in our bones and teeth
- Sodium - in salt
- Chlorine - in drinking water and swimming pools
- Aluminum - used in cans and foil
- Copper - in wiring and plumbing
- Iron - in foods and construction materials
- Zinc - in galvanization and supplements
- Iodine - added to salt for thyroid healthLearning_The_Periodic_Table.ppt



Learning_The_Periodic_Table.pptJane Doe
╠²
Here are some common elements we use every day:
- Carbon - found in pencils, food, plastics
- Oxygen - we breathe it
- Hydrogen - found in water
- Nitrogen - in the air we breathe
- Calcium - in our bones and teeth
- Sodium - in salt
- Chlorine - in drinking water and swimming pools
- Aluminum - used in cans and foil
- Copper - in wiring and plumbing
- Iron - in foods and construction materials
- Zinc - in galvanization and supplements
- Iodine - added to salt for thyroid health3 130821054737-phpapp01



3 130821054737-phpapp01Chen2020
╠²
Mendeleev arranged the elements in order of increasing atomic mass in a periodic table. He noticed that elements with similar properties fell into recurring patterns, allowing him to predict properties of undiscovered elements. Later, Moseley arranged elements by atomic number, better reflecting their properties. The periodic table organizes elements into metals, nonmetals, and metalloids, which have characteristic physical and chemical properties depending on their group and period.The Periodic Table 



The Periodic Table Melinda MacDonald
╠²
Mendeleev arranged the elements in order of increasing atomic mass in a periodic table. He noticed that elements with similar properties appeared to repeat periodically. This allowed him to predict properties of undiscovered elements and correct properties of known elements. Later, Moseley arranged elements by atomic number, solidifying the periodic law. The periodic table organizes elements into metals, nonmetals, and metalloids and is still used to predict properties of new elements.Learning_The_Periodic_Table.ppt



Learning_The_Periodic_Table.pptLAVENAAMORA
╠²
Here are some common elements we use every day:
- Carbon - found in pencils, food, plastics
- Oxygen - we breathe it
- Hydrogen - found in water
- Nitrogen - found in air and proteins
- Calcium - found in bones and milk
- Sodium - found in salt
- Chlorine - used to purify water
- Aluminum - used in cans and foil
- Iron - found in foods and used to make steel
- Copper - used in wiring and plumbing
- Zinc - found in vitamins and skin creams
- Iodine - added to salt to prevent deficiencies
- Gold - used in jewelry
- SilverArranging the elements gr.7 2018



Arranging the elements gr.7 2018Ruba Salah
╠²
This document provides information about the periodic table, including its history and key features. It discusses how Dmitry Mendeleev discovered a pattern among the elements based on their atomic mass, which led to the creation of the periodic table. Later, Henry Moseley determined that arranging elements by atomic number (number of protons) better fit the observed patterns. The document then describes the main components of the periodic table including periods and groups, and provides examples of the types of information contained in each square. It also defines metals, non-metals and metalloids, and their distinguishing properties.Atomic Number.pptSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS...



Atomic Number.pptSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS...redcarbin
╠²
Here are some common elements we use every day:
- Carbon - found in pencil lead, food, and our bodies
- Oxygen - we breathe it
- Hydrogen - found in water
- Nitrogen - makes up most of the air we breathe
- Calcium - found in bones and milk
- Sodium - found in salt
- Chlorine - used to purify water
- Aluminum - used in cans and foil
- Copper - used in wiring and plumbing
- Iron - found in many foods and our blood
- Zinc - supports immune function and found in ointments
- Iodine - essential for thyroid function and added to saltChem115 unit1



Chem115 unit1klhuisinga
╠²
This document provides an overview of chemistry. It defines chemistry as the study of matter, its properties, and the changes it undergoes. It discusses the major areas of chemistry including biochemistry, organic chemistry, inorganic chemistry, analytic chemistry, and physical chemistry. It also covers key concepts such as the classification of matter into elements, compounds, mixtures, and states of matter. The periodic table is introduced as a way to organize the known elements. Metals, nonmetals, and metalloids are classified based on their properties. Physical and chemical properties and changes are distinguished.Atomic Numberrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrr



Atomic Numberrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrremgreyz11
╠²
jjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjRecently uploaded (20)
Functional Muscle Testing of Facial Muscles.pdf



Functional Muscle Testing of Facial Muscles.pdfSamarHosni3
╠²
Functional Muscle Testing of Facial Muscles.pdfYear 10 The Senior Phase Session 3 Term 1.pptx



Year 10 The Senior Phase Session 3 Term 1.pptxmansk2
╠²
Year 10 The Senior Phase Session 3 Term 1.pptxAzure Data Engineer Interview Questions By ScholarHat



Azure Data Engineer Interview Questions By ScholarHatScholarhat
╠²
Azure Data Engineer Interview Questions By ScholarHatBISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAH



BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHcoacharyasetiyaki
╠²
BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHNUTRITIONAL ASSESSMENT AND EDUCATION - 5TH SEM.pdf



NUTRITIONAL ASSESSMENT AND EDUCATION - 5TH SEM.pdfDolisha Warbi
╠²
NUTRITIONAL ASSESSMENT AND EDUCATION, Introduction, definition, types - macronutrient and micronutrient, food pyramid, meal planning, nutritional assessment of individual, family and community by using appropriate method, nutrition education, nutritional rehabilitation, nutritional deficiency disorder, law/policies regarding nutrition in India, food hygiene, food fortification, food handling and storage, food preservation, food preparation, food purchase, food consumption, food borne diseases, food poisoningInventory Reporting in Odoo 17 - Odoo 17 Inventory App



Inventory Reporting in Odoo 17 - Odoo 17 Inventory AppCeline George
╠²
This slide will helps us to efficiently create detailed reports of different records defined in its modules, both analytical and quantitative, with Odoo 17 ERP.Hannah Borhan and Pietro Gagliardi OECD present 'From classroom to community ...



Hannah Borhan and Pietro Gagliardi OECD present 'From classroom to community ...EduSkills OECD
╠²
Hannah Borhan, Research Assistant, OECD Education and Skills Directorate and Pietro Gagliardi, Policy Analyst, OECD Public Governance Directorate present at the OECD webinar 'From classroom to community engagement: Promoting active citizenship among young people" on 25 February 2025. You can find the recording of the webinar on the website https://oecdedutoday.com/webinars/
How to create security group category in Odoo 17



How to create security group category in Odoo 17Celine George
╠²
This slide will represent the creation of security group category in odoo 17. Security groups are essential for managing user access and permissions across different modules. Creating a security group category helps to organize related user groups and streamline permission settings within a specific module or functionality.Azure Administrator Interview Questions By ScholarHat



Azure Administrator Interview Questions By ScholarHatScholarhat
╠²
Azure Administrator Interview Questions By ScholarHatUnit 1 Computer Hardware for Educational Computing.pptx



Unit 1 Computer Hardware for Educational Computing.pptxRomaSmart1
╠²
Computers have revolutionized various sectors, including education, by enhancing learning experiences and making information more accessible. This presentation, "Computer Hardware for Educational Computing," introduces the fundamental aspects of computers, including their definition, characteristics, classification, and significance in the educational domain. Understanding these concepts helps educators and students leverage technology for more effective learning.One Click RFQ Cancellation in Odoo 18 - Odoo ║▌║▌▀Żs



One Click RFQ Cancellation in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
In this slide, weŌĆÖll discuss the one click RFQ Cancellation in odoo 18. One-Click RFQ Cancellation in Odoo 18 is a feature that allows users to quickly and easily cancel Request for Quotations (RFQs) with a single click.Meeting the needs of modern students?, Selina McCoy



Meeting the needs of modern students?, Selina McCoyEconomic and Social Research Institute
╠²
NAPD Annual Symposium
ŌĆ£Equity in our Schools: Does the system deliver for all young people?ŌĆØHelping Autistic Girls Shine Webinar ║▌║▌▀Żs



Helping Autistic Girls Shine Webinar ║▌║▌▀ŻsPooky Knightsmith
╠²
For more information about my speaking and training work, visit: https://www.pookyknightsmith.com/speaking/Dot NET Core Interview Questions PDF By ScholarHat



Dot NET Core Interview Questions PDF By ScholarHatScholarhat
╠²
Dot NET Core Interview Questions PDF By ScholarHatDr. Ansari Khurshid Ahmed- Factors affecting Validity of a Test.pptx



Dr. Ansari Khurshid Ahmed- Factors affecting Validity of a Test.pptxKhurshid Ahmed Ansari
╠²
Validity is an important characteristic of a test. A test having low validity is of little use. Validity is the accuracy with which a test measures whatever it is supposed to measure. Validity can be low, moderate or high. There are many factors which affect the validity of a test. If these factors are controlled, then the validity of the test can be maintained to a high level. In the power point presentation, factors affecting validity are discussed with the help of concrete examples.Administrative bodies( D and C Act, 1940



Administrative bodies( D and C Act, 1940P.N.DESHMUKH
╠²
These presentation include information about administrative bodies such as D.T.A.B
CDL AND DCC, etc.How to Configure Recurring Revenue in Odoo 17 CRM



How to Configure Recurring Revenue in Odoo 17 CRMCeline George
╠²
This slide will represent how to configure Recurring revenue. Recurring revenue are the income generated at a particular interval. Typically, the interval can be monthly, yearly, or we can customize the intervals for a product or service based on its subscription or contract. Elements and compounds (1) (1)
- 1. Elements and compounds By Sidra, Manahil and Rida of class 7N
- 2. Differences between Elements and Compounds Elements ŌĆó Elements are those substances which cannot be further subdivided in to other substances. ŌĆó Examples are Sodium, iron, oxygen and many more. Compounds ŌĆó Compounds are those substances that consist of few or more elements which have been chemically combined. ŌĆó Examples: Water, Sodium Chloride.
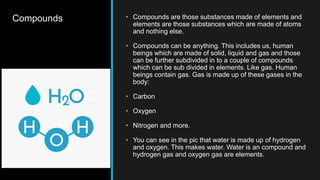
- 3. Compounds ŌĆó Compounds are those substances made of elements and elements are those substances which are made of atoms and nothing else. ŌĆó Compounds can be anything. This includes us, human beings which are made of solid, liquid and gas and those can be further subdivided in to a couple of compounds which can be sub divided in elements. Like gas. Human beings contain gas. Gas is made up of these gases in the body: ŌĆó Carbon ŌĆó Oxygen ŌĆó Nitrogen and more. ŌĆó You can see in the pic that water is made up of hydrogen and oxygen. This makes water. Water is an compound and hydrogen gas and oxygen gas are elements.
- 4. Elements ŌĆó Elements are those substances which cannot be further subdivided by even chemical reactions. ŌĆó A element is a pure substance only formed by a single type of atom. ŌĆó There are 112 elements which of 96 are natural while only 16 of them are man made. ŌĆó The 2 most abundant elements of this universe are known as helium and hydrogen.
- 5. The periodic table ŌĆó The periodic table is a table of elements. ŌĆó This table is divided and coloured in to elements that are metallic and non metallic.
- 6. Elements and there chemical symbols ŌĆó You can see in the table later that there is a appropriate chemical symbol for every element. ŌĆó All these elements can be represented easily by these chemical symbols so that it is easy to read them out. ŌĆó These can be also said using the german or latin name to name things easily when the English name is already taken. Just like for iron. We say ŌĆśFeŌĆÖ because the name in german or latin is ferrum as iron name is already taken for iodine.
- 7. A few symbol examples.
- 8. You can view the table below.
- 9. Reading the periodic table ŌĆó The periodic table is read in a certain manner. ŌĆó The top number for each element is its atomic number. This is the number of protons in each atom of that element. ŌĆó The one-letter or two-letter symbol in each tile is the element symbol. The symbol is an abbreviation for the full element name. Element symbols make it much easier for chemists to write chemical formulas and equations. ŌĆó The bottom number in each element tile is the atomic weight or atomic mass. This value is the average mass of atoms of that element that occur naturally. ŌĆó Rows of elements are called periods. The period number of an element signifies the highest unexcited energy level for an electron in that element. The number of elements in a period increases as you move down the periodic table because there are more sublevels per level as the energy level of the atom increases. ŌĆó Columns of elements help define element groups. Elements within a group share several common properties.
- 10. Metals ŌĆó This is a few examples to recognize metals: ŌĆó usually solid at room temperature (except mercury) ŌĆó metallic-looking ŌĆó hard ŌĆó shiny ŌĆó good conductors of heat and electricity
- 11. Non metals ŌĆó These are a few examples to recognize non metals: ŌĆó often form brittle solids ŌĆó lacking in metallic luster ŌĆó poor conductors of heat and electricity
- 12. Pie chart
- 13. Uses of elements ŌĆó Elements are used to make compounds and compounds are used to make NEARLY EVERYTHING. ŌĆó Elements help in making chemical reactions to make more elements ŌĆó Elements are the main building of science and science is developing because of it. ŌĆó We breath from oxygen. Other elements are used to make other things like water when oxygen is mixed with hydrogen.
- 14. Examples of uses of elements: ŌĆó Chalk: made of; calcium,carbon,oxygen. ŌĆó Sugar: carbon, hydrogen, oxygen ŌĆó Polythene: carbon, hydrogen
- 15. Gases in the human body
- 16. Some tidbits you should now ŌĆó The Russian scientists , Dmitri Ivanovich Mendeleev (1834-1907), was the first to arrange the 63 known elements during his time according to their properties in the periodic table . He also predicted the existence and properties of new elements which were yet to be discovered.
- 17. Summary of the chapter ŌĆó An element is a substance which cannot be broken down into simpler substances by chemical reactions ŌĆó A chemical reaction is a process in which new substances are formed . ŌĆó There are more than 100 elements , about 90 of which occur naturally . All other materials are made from elements hydrogen , oxygen ,helium , carbon , silicon and many more are examples of elements ŌĆó Elements can be classified by being non metal or metal elements have many uses and applications in daily life . ŌĆó The uses and applications of elements depend on their properties.
- 18. Trivia time Which one of the following is NOT an element: There are ____________ elements a) Magnesium a) 120 b) Helium b) 168 c) Aspirin c) 105 d) Sodium d) 112 Water is made up of oxygen and ________ which one of the chemical symbol stands for ŌĆ£hydrogenŌĆØ a) Carbon dioxide a) HE b) Hydrogen b) H c) Nitrogen c) AU d) Argon d) HN
- 19. Thank you for listening By Manahil, Sidra , Rida of class 7N you can contact us at: Sidraashraf2008@gmail.com usmanimanahil09@gmail.com Ridatariq1616@gmail.com



