Embryology of Skin - Epidermal Development , Development of the specialized cells of the epidermis
- 1. Embryology of the Skin Presented by: Alanoud Al-Marzoug R2
- 2. Outline Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
- 3. • During the 3rd week after fertilization, the human embryo undergoes gastrulation , that results in the formation of the three embryonic germ layers : • Ectoderm. • Mesoderm. • Endoderm. INTRODUCTION
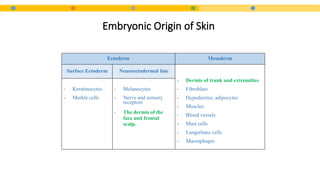
- 4. Embryonic Origin of Skin Ectoderm Mesoderm Surface Ectoderm Neuroectodermal fate - Dermis of trunk and extremities - Fibroblast - Hypodermis; adipocytes - Muscles - Blood vessels - Mast cells - Langerhans cells - Macrophages - Keratinocytes - Merkle cells - Melanocytes - Nerve and sensory receptors - The dermis of the face and frontal scalp.
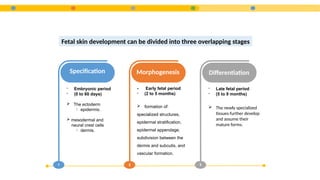
- 5. 1 Specification - Embryonic period - (0 to 60 days)  The ectoderm ïƒ epidermis.  mesodermal and neural crest cells ïƒ dermis. 2 Morphogenesis - Early fetal period - (2 to 5 months)  formation of: specialized structures, epidermal stratification, epidermal appendage, subdivision between the dermis and subcutis, and vascular formation. 3 Differentiation - Late fetal period - (5 to 9 months)  The newly specialized tissues further develop and assume their mature forms. Fetal skin development can be divided into three overlapping stages
- 6. Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
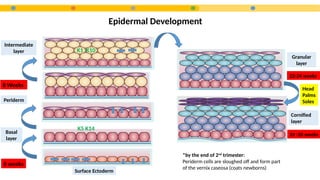
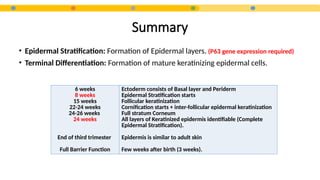
- 8. Epidermal Development Surface Ectoderm Basal layer Periderm Intermediate layer Granular layer Cornified layer 6 weeks 8 Weeks K5 K14 K1 K10 22-24 weeks 24 -26 weeks Head Palms Soles *by the end of 2nd trimester: Periderm cells are sloughed off and form part of the vernix caseosa (coats newborns)
- 9. Summary • Epidermal Stratification: Formation of Epidermal layers. (P63 gene expression required) • Terminal Differentiation: Formation of mature keratinizing epidermal cells. 6 weeks 8 weeks 15 weeks 22-24 weeks 24-26 weeks 24 weeks End of third trimester Full Barrier Function Ectoderm consists of Basal layer and Periderm Epidermal Stratification starts Follicular keratinization Cornification starts + inter-follicular epidermal keratinization Full stratum Corneum All layers of Keratinized epidermis identifiable (Complete Epidermal Stratification). Epidermis is similar to adult skin Few weeks after birth (3 weeks).
- 10. Clinical Relevance • Collodion Baby: Encased in a Taut, shiny, transparent membrane that is formed by aberrant stratum corneum .
- 11. • After shedding the Collodion membrane, most of these infant manifest with: Lamellar Ichthyosis or Congenital Ichthyosiform Erythroderm • Mood of inheritance? • Gene defect? • Clinical Presentation? AR TGM1 gene (transglutaminase deficiency) or ABCA12 (ATP binding cassette A12) 1- Collodion membrane at birth with underlying erythroderma → evolves to thick dark scales with flexural involvement. 2- Associated ectropion, eclabium, scarring alopecia PPK, heat intolerance (heat stroke),hypernatremia.
- 12. Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
- 13. All melanocytes are functional and in place at birth  Unique Migration: Trunk: dorsolaterally then ventrally around the trunk to ventral midline Scalp and face: Anteriorly Extremities: distally Melanocytes First Identified within the epidermis 2 months (50 days) Melanin Production 4 months (Fitz 3 months) Trasfer Melanosomes to Keratinocytes 6 months
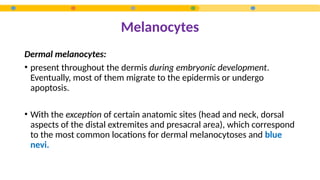
- 14. Melanocytes Dermal melanocytes: • present throughout the dermis during embryonic development. Eventually, most of them migrate to the epidermis or undergo apoptosis. • With the exception of certain anatomic sites (head and neck, dorsal aspects of the distal extremites and presacral area), which correspond to the most common locations for dermal melanocytoses and blue nevi.
- 15. Clinical Relevance • Mood of inheritance? • Gene defect? • Clinical Presentation? White forelock (poliosis) AD C-kit gene mutation White forelock (poliosis) Irregularly-shaped leukoderma favoring anterior trunk, extremities, forehead (spares hands, feet, hips, shoulders), otherwise healthy. Piebaldism
- 16. Markers: Langerhans cells First Identified within the epidermis 6-7 weeks (40 days) Fully developed 14 weeks • CD45 • CD207 (Langrein) • CD1a • S100 • Vimentin S100 expression Eslamc +S100 Eccrine Schwan Langerhan Adipocytes Melanocytes Chondrocytes
- 17. • Highly innervated neuroendocrine cells involved in mechanoreception. • First identified in palmo-planter epidermis: 8-12 weeks EGA. • Particularly dense over volar skin. • Markers: Merkel cells  CK20  Chromogranin  Somatostatin  Calcitonin  Synaptophysin  Vasoactive peptide  Neuron-specific Enolase
- 18. Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
- 19. • The embryonic tissue that forms the dermis depends on the specific body site. DERMIS Dermis of the face and anterior scalp Neural crest ectoderm Dermis of the back dermomyotome of the embryonic somite Dermis of extremities and ventral trunk lateral plate mesoderm
- 20. DERMIS • The ratio of collagen III to collagen I is 3:1 (the reverse of adult dermis). • Dermal vasculature: by the end of the first trimester, but it undergoes extensive remodeling in utero and is not fully mature until after birth. • Nerve networks: by the mid to late first trimester, and they tend to follow the vascular pattern. • Accumulation of subcutaneous fat begins during the second trimester and continues through the third trimester, when distinct lobules separated by fibrous septae are formed. 6–8 weeks Dermal fibroblasts 9 weeks Demarcation between dermis and underlying skeletal condensations 12- 15 weeks Distinguish Papillary from reticular dermis 22 – 24 weeks Elastic fibers first detected
- 21. Clinical Relevance Ehler Danlos Syndrome
- 22. Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
- 23. • As development progresses, the flat embryonic DEJ acquires the rete ridges and dermal papillae that characterize the adult DEJ. Dermal Epidermal Junction 8 weeks Skin specific components to the DEJ start to appear (Laminin 1, Collagen 4, Heparin sulfate) 12 weeks Almost all structures of the mature DEJ are in place
- 24. Epidermal Development Development of the specialized cells of the epidermis Development of the Dermis and Subcutis Development of the dermal- epidermal junction Development of the Skin Appendages  Melnocytes  Langerhans cells  Merckle cells  Hair Follicle  Sebaceous Gland  Nails  Sweat Glands (APOCRINE, ECCRINE)
- 25. • Skin appendages (Hair, Nails and Sebaceous Glands ) have both  Epidermal and dermal components that are critical in embryogenesis. SKIN APPENDAGES
- 26. Hair Follicle Development • Follicle formation is initiated by signals from the dermis that direct the embryonic epidermis to form focal thickenings, called placodes. • Placodes are first seen on the scalp and face • They subsequently develop in a caudal and then in a ventral direction • The epidermal placodes instruct the underlying dermal cells to condense and form the presumptive dermal papilla. • The dermal papilla then directs the keratinocytes of the placode to proliferate and extend deeper into the dermis, thereby forming the hair germ. • The base of the developing hair follicle surrounds the presumptive dermal papilla, forming the hair peg. First dermal signal
- 27. Hair Follicle Development The superficial portion of the developing hair follicle has two distinct bulges: • Superficial bulge ïƒ sebaceous gland. • Deeper bulge ïƒ The insertion point of the future arrector pili muscle ïƒ The location of the presumptive follicular stem cells
- 28. • The first hairs that appear, lanugo (downy hair) are fine, soft and lightly pigmented. • Begins to appear by the end of 12th week and is plentiful by the 17th -20th week. • They help to hold the vernix caseosa on the skin. • Lanugo is replaced during the perinatal period by coarser hair. Hair Follicle Development
- 29. Hair Follicle Development • Hair follicles undergo further maturation during the second trimester, forming seven concentric cell layers.
- 30. Hair Follicle Development 10 – 11 weeks Placodes (first seen on scalp and face) 12 – 14 weeks Hair Peg 12 – 24 weeks 7 concentric hair follicle layers 19 – 21 weeks Hair canal fully formed (visible hair) 24 – 28 weeks The hairs continue to grow (anagen > catagen > telogen) After birth The third hair cycle is initiated (asynchronous hair cycle)
- 31. • Sonic hedgehog (SHH): a signaling molecule secreted by cells of the developing hair follicles, is required for the maturation of the dermal papilla and for the progression of the follicle placode to the hair bulb stage. • SHH is also critical for the transition from the telogen to anagen during postnatal hair cycling. Hair Follicle Development
- 32. • First seen: 13–16 weeks GA as the most superficial bulge on the developing hair follicle. • Maternal hormones contribute to sebaceous gland hypertrophy and increased synthesis and secretion of sebum during the second and third trimesters. (start secretion intrauterine) SEBACEOUS GLANDS
- 33. • Sweat glands are of two types: 1. Eccrine:  located throughout most of the body.  The ducts open into the skin surface.  Begin to function shortly after birth (Do Not function intrauterine) 1. Apocrine:  Confined to the axilla, areola, and pubic and perineal regions.  Ducts open into the upper part of hair follicles superficial to the opening of the sebaceous glands.  Function transiently during the 3rd trimester and subsequently becomes quiescent in the neonate.  Begin to function during puberty. SWEAT GLANDS
- 34. SWEAT GLANDS 55 – 65 days Eccrine glands begin to develop on the volar surfaces of the hands and feet, beginning as mesenchymal pads 12 -14 weeks parallel ectodermal ridges are induced which overlay these pads. 5th Month • Dermatographics (fingerprints) seen on digit tips • Interfollicular eccrine and apocrine glands begin to bud 7th Month The cells of the apocrine glands become distinguishable • Palmoplantar eccrine sweat gland start to develop during 1st trimester and fully developed in 2nd trimester. • Like sebaceous glands, apocrine glands typically arise from the upper portion of a hair follicle, unlike to the inter-follicular eccrine gland which originate independantly.
- 35. 9 weeks Nail apparatus developed from epidermis dorsal tip of the digit 12 weeks Primordial nail matrix 15 weeks Nail matrix completely developed 5 months Nail plate completely covering nail bed NAILS
- 36. Quiz
- 37. The embryonic periderm becomes part of the? a. Vernix caseosa b. stratum corneum c. stratum basale d. dermis e. hair follicle
- 38. Embryologically, epidermal stratification begins at approximately what estimated gestational age? a. 4 weeks b. 8 weeks c. 12 weeks d. 16 weeks e. 20 weeks
- 39. Keratinocytes are derived from which of the following? a. Endoderm b. Mesoderm c. Ectoderm d. Neural Crest e. Bone marrow precursors
- 40. • Dermatology by Bolognia J. 4th Ed. • Fitzpatrick’s dermatology in general medicine. 8th Ed. References:
Editor's Notes
- #8: Vernix caseosa function: Protects the developing skin from constant exposure to amniotic fluid with its urine content during the fetal period Facilitates birth because of its slippery nature.
- #11: - What other types of icthyosis present with Collodion membrane? HI, Netherton What other type of icthyosis can result from ABCA12? HI
- #15: c-kit gene mutation (proto-oncogene, tyrosine-kinase receptor Family)
- #16: S100 expression Eslamc +S100 Eccrine Schwan Langerhan Adipocytes Melanocytes Chondrocytes