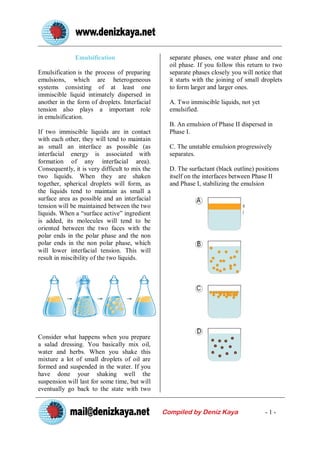
Emulsification
- 1. Emulsification separate phases, one water phase and one oil phase. If you follow this return to two Emulsification is the process of preparing separate phases closely you will notice that emulsions, which are heterogeneous it starts with the joining of small droplets systems consisting of at least one to form larger and larger ones. immiscible liquid intimately dispersed in another in the form of droplets. Interfacial A. Two immiscible liquids, not yet tension also plays a important role emulsified. in emulsification. B. An emulsion of Phase II dispersed in If two immiscible liquids are in contact Phase I. with each other, they will tend to maintain as small an interface as possible (as C. The unstable emulsion progressively interfacial energy is associated with separates. formation of any interfacial area). Consequently, it is very difficult to mix the D. The surfactant (black outline) positions two liquids. When they are shaken itself on the interfaces between Phase II together, spherical droplets will form, as and Phase I, stabilizing the emulsion the liquids tend to maintain as small a surface area as possible and an interfacial tension will be maintained between the two liquids. When a ˇ°surface activeˇ± ingredient is added, its molecules will tend to be oriented between the two faces with the polar ends in the polar phase and the non polar ends in the non polar phase, which will lower interfacial tension. This will result in miscibility of the two liquids. Consider what happens when you prepare a salad dressing. You basically mix oil, water and herbs. When you shake this mixture a lot of small droplets of oil are formed and suspended in the water. If you have done your shaking well the suspension will last for some time, but will eventually go back to the state with two Compiled by Deniz Kaya -1-