Emulsions
Download as PPTX, PDF17 likes15,879 views
Emulsion definition, types, characteristics, preparation, emulsifying agents, Identification tests, advantages, disadvantages and pharmaceutical application.
1 of 23
Downloaded 177 times


















Recommended
emulsion



emulsionRavikumar Patil
Ěý
This document discusses emulsions. It defines an emulsion as a dispersion of small globules of one liquid distributed throughout another immiscible liquid. Emulsions are classified based on the dispersed phase as oil-in-water or water-in-oil, and based on droplet size as macroemulsions or microemulsions. Emulsifying agents are substances that stabilize emulsions by forming films at the liquid interfaces. Various natural, semi-synthetic, and synthetic agents are described. Methods for preparing emulsions include dry gum, wet gum, and bottle methods. Factors that cause emulsion instability like cracking and creaming are also outlined.Emulsion



EmulsionAkshayAkotkar
Ěý
1) An emulsion is an unstable mixture of two immiscible liquids, where one liquid is dispersed as globules in the other liquid. Emulsions can be O/W (oil in water) or W/O (water in oil) types.
2) Pharmaceutical emulsions are used to deliver unpleasant tasting drugs, provide slow release of water-soluble drugs, and enhance absorption of oil-soluble drugs.
3) The key steps in formulating an emulsion are selecting an emulsifying agent based on its HLB value, adding preservatives and antioxidants, and using methods like trituration or the bottle method to prepare the emulsion.Monophasic liquids



Monophasic liquidsRavikumar Patil
Ěý
This document provides information on various types of monophasic liquid dosage forms, including their definitions, advantages, disadvantages, examples, and typical formulation methods. It discusses gargles, mouthwashes, throat paints, ear drops, nasal drops, syrups, elixirs, liniments, and lotions. For each type, it provides a brief description of its use and purpose as well as an example formulation and method.Stability of emulsion



Stability of emulsionShikha Thakur
Ěý
This document discusses factors that can cause instability in emulsions over time during storage. The three main changes that can occur are cracking, creaming, and phase inversion. Cracking is the separation of phases and can result from changes in emulsifying agents, solvents, microbes, temperature, or creaming. Creaming is the upward movement of dispersed globules, which depends on globule size, density differences, viscosity, and storage temperature. Phase inversion is a change from one emulsion type to the other, such as oil-in-water to water-in-oil, brought on by electrolytes, phase volume ratios, temperature, or emulsifying agents. Proper packaging, labeling, and storage conditions can help promote emulsionEmulsion



EmulsionAmit M Gupta
Ěý
An emulsion is an unstable mixture of two immiscible liquids stabilized by an emulsifying agent. Emulsions have various pharmaceutical applications including oral and topical drug delivery. The type of emulsion (e.g. oil-in-water, water-in-oil) depends on the relative solubility of the emulsifying agent. Emulsions can be prepared using different methods such as the dry gum, wet gum, or bottle methods. Drugs can be incorporated into emulsions during or after emulsion formation.Pharmaceutical suppositories



Pharmaceutical suppositoriesJabir Jabir
Ěý
A suppository is a drug delivery system that is inserted into the rectum (rectal suppository), vagina (vaginal suppository) or urethra (urethral suppository), where it dissolves or melts and is absorbed into the blood stream. They are used to deliver both systemically and locally acting medications.Ch.13 part 4 liniment, collodion, glycerite



Ch.13 part 4 liniment, collodion, glyceriteMalou Mojares
Ěý
embrocation, liniment, collodion, pyroxillin, glycerite, starch glycerite, oleovitamin, scott's emulsion, toothache dropsPharmaceutical Emulsion



Pharmaceutical EmulsionMirza Salman Baig
Ěý
Pharmaceutical Emulsions are thermodynamically unstable mixtures of two immiscible liquids stabilized by an emulsifying agent. They can be oil-in-water (O/W) or water-in-oil (W/O) emulsions depending on the dispersed and continuous phases. Emulsifying agents like surfactants, hydrocolloids, and solid particles form protective films around droplets and increase viscosity to prevent coalescence. Stability issues include creaming, cracking, and phase inversion. Methods to enhance stability are reducing droplet size, increasing viscosity, using emulsifying agents, and controlling storage temperature.Elixirs



ElixirsSadaqat Ali
Ěý
Elixirs are clear, sweetened alcoholic solutions intended for oral use. They contain 10-12% alcohol which helps dissolve ingredients. Elixirs differ from syrups in that alcohol is always present in elixirs and they remain clear while syrups can contain dyes. Common types of elixirs include simple non-medicated elixirs and medicated elixirs containing active ingredients. Elixirs are prepared by separately dissolving water and alcohol soluble components before combining the solutions and adding excipients like sweeteners, flavors, and preservatives.Pharmaceutics - emulsions



Pharmaceutics - emulsionsAreej Abu Hanieh
Ěý
An emulsion is an unstable mixture of two immiscible liquids, where one liquid is dispersed as globules in the other liquid. Emulsions can be oil-in-water or water-in-oil depending on the continuous and dispersed phases. Surfactants are needed to stabilize emulsions by lowering surface tension at the interface between the liquids. The document discusses different types of emulsifiers including surface-active agents, hydrocolloids, and solid particles that stabilize emulsions through monomolecular or multimolecular film formation. It also covers emulsion characterization, applications in pharmaceutical products, and factors affecting emulsion stability.Pharmaceutical Suspensions and Emulsions



Pharmaceutical Suspensions and EmulsionsPallavi Kurra
Ěý
A detailed description about Pharmaceutical Suspensions and Emulsions or Disperse systems was given in this pptSuspensions



SuspensionsSaif Khan
Ěý
The document discusses pharmaceutical suspensions. A suspension is a coarse dispersion where an insoluble internal phase is dispersed uniformly throughout an external phase. Reasons for formulating suspensions include insolubility of the drug, masking bitter taste, increasing stability, and achieving controlled drug release. Common types of suspensions include antacids, antibiotics, analgesics, anthelmintics, and antifungals. Suspending agents are used to prevent sedimentation and ensure uniform dosing. Preparation involves grinding the insoluble drug into a paste and incorporating suspending agents before making up the final volume. Advantages include improved stability and bioavailability for some drugs, while disadvantages include issues with physical stability and accurate dosing.Monophasic liquid dosage forms part 1 



Monophasic liquid dosage forms part 1 Prof. Sandhya Lanke/ Sudrik
Ěý
Monophasic liquid dosage forms are liquid preparations containing two or more components in a single phase system that form a true solution. They have advantages like being easier to swallow, increasing drug absorption rates, and allowing flexible dosing. However, they can also be unstable, have unpleasant tastes, and require accuracy in dosing. The document further classifies liquid dosage forms and discusses mixtures, syrups, elixirs, and other types used internally or externally.Semisolid dosage -Ointments, Pate,Jellies



Semisolid dosage -Ointments, Pate,JelliesRavikumar Patil
Ěý
This document provides information about semisolid dosage forms such as ointments, pastes, and jellies. It defines semisolids as topical dosage forms used for therapeutic, protective, or cosmetic purposes. The key ingredients in semisolids include a base, preservatives, humectants, antioxidants, emulsifiers, gelling agents, permeation enhancers, and buffers. The document discusses the ideal properties of bases and lists common bases such as petrolatum, lanolin, and polyethylene glycol. It also covers the advantages and disadvantages of semisolid dosage forms.Emusification



Emusificationhassanraza406
Ěý
The document defines an emulsion as a mixture of two or more liquids that are normally immiscible. It then discusses the internal and external phases of emulsions, types of emulsions based on dispersed phase and size, advantages and disadvantages, identification tests, emulsifying agents, theories of emulsification, and factors that contribute to emulsion stability like interfacial tension. In summary, the key topics covered are the definition of emulsions, classification based on phase and size, tests to identify emulsion types, role of emulsifying agents, and theories to explain emulsion stabilization mechanisms.LIQUID DOSAGE FORMS.pptx



LIQUID DOSAGE FORMS.pptxSUJITHA MARY
Ěý
Liquid dosage forms: Advantages and disadvantages of liquid dosage forms. Excipients used in formulation of liquid dosage forms. Solubility enhancement techniquesInstability Of Emulsions.pptx



Instability Of Emulsions.pptxYashThorat20
Ěý
The document discusses various types of instability that can occur in pharmaceutical emulsions, including flocculation, coalescence, creaming, breaking, deterioration by microorganisms, and physical/chemical changes. Flocculation involves droplets aggregating into larger units while coalescence occurs when droplets merge to form even larger droplets. Creaming is the rising of dispersed globules, and breaking refers to the complete separation of emulsion phases. Proper formulation, storage conditions, and addition of preservatives can help prevent these instability issues.Monophasic liquid dosage forms part 3



Monophasic liquid dosage forms part 3Prof. Sandhya Lanke/ Sudrik
Ěý
In this presentation viewers will able to learn about liquids for internal administration such as syrups, elixirs and linctus.Suspending agents



Suspending agentsSILVI SINGH
Ěý
This document discusses different types of suspending agents used in pharmaceutical formulations. It classifies suspending agents into polysaccharides, inorganic salts, and synthetic compounds. Some examples of polysaccharides agents include acacia, tragacanth, and starches. Common inorganic salts are bentonite, aluminum magnesium silicate, and aluminum hydroxide. Synthetic agents include carbomers and colloidal silicon dioxide. Suspending agents help stabilize suspensions by increasing viscosity and slowing particle sedimentation according to Stokes' law. They prevent caking and can be resuspended with agitation.Monophasic liquid dosage forms part 4



Monophasic liquid dosage forms part 4Prof. Sandhya Lanke/ Sudrik
Ěý
In this presentation viewers will able to learn about liquids for external use such as liniments and lotions, liquids for oral cavity such as mouthwash, throat paints and gargles.Test for identification of type of emulsion



Test for identification of type of emulsionSantuMistree4
Ěý
Four tests are used to identify oil-in-water (O/W) and water-in-oil (W/O) emulsions: the dilution test, dye test, conductivity test, and fluorescence test. The dilution test identifies the emulsion type based on whether it dilutes easily with water or oil. The dye test observes emulsion droplets under a microscope after adding an oil-soluble dye. If the continuous phase is colored and droplets are clear, it is a W/O emulsion; if droplets are colored and the continuous phase is clear, it is an O/W emulsion. The conductivity test uses electrodes - if a bulb glows, it is an O/W emulsion, and if not, it is aStability of suspensions



Stability of suspensionsAbdelrhman abooda
Ěý
This document discusses stability factors and applications of pharmaceutical suspensions. It notes that small particle size, increasing viscosity, and maintaining optimal temperature contribute to suspension stability. Suspensions are used for insoluble drugs, to improve drug stability, and to mask unpleasant tastes. Key factors for stability include particle size, viscosity, temperature, surfactants, hydrophilic colloids, solvents, and proper mixing procedures.Emulsions



EmulsionsIqra Zulfiqar Ali Rajput
Ěý
This document provides an overview of pharmaceutical emulsions. It defines emulsions as dispersions of one liquid in another immiscible liquid, stabilized by an emulsifying agent. The key topics covered include the classification of emulsions as oil-in-water or water-in-oil, theories of emulsification, common emulsifying agents like surfactants and hydrocolloids, and factors affecting the stability of emulsions such as flocculation and creaming. Pharmaceutical applications of emulsions include lotions, creams, and ointments.Monophasic liquid dosage form ppt



Monophasic liquid dosage form pptmeenakharwade1
Ěý
This document provides information on various monophasic liquid dosage forms including gargles, mouthwashes, throat paints, and syrups. It discusses the components, advantages, disadvantages, and methods of preparation for each type. Gargles are aqueous solutions used to treat throat infections that are prepared by dissolving ingredients in solvents. Mouthwashes are solutions used for oral hygiene that can be cosmetic or therapeutic. Throat paints are viscous liquids applied to the mouth and throat to treat infections. Syrups are concentrated sugar solutions that can also contain medication, providing a pleasant way to administer liquid drugs. The document outlines the typical ingredients and formulations for each monophasic liquid dosage form.Semisolid dosage forms: Paste and Jellies



Semisolid dosage forms: Paste and JelliesParag Jain
Ěý
Difference between ointments and paste, preparation of paste, preservatives, jellies, and preparations, poultices Semisolid dosage forms ppt



Semisolid dosage forms pptPranatiChavan
Ěý
Semisolid dosage forms are neither solid nor liquid, however, they are a combination or mixture of both, and they used for both local and systemic effects. Pharmaceutical semisolid dosage forms such as creams, ointments, gels, suppositories, and paste are used for topical application. Semisolid dosage forms are intended used as drug carriers that are transported topically through the skin, buckle tissue, rectal tissue, outer ear lining nasal mucosa, urethral membrane, vagina, and cornea. The semisolid may adhere adequately before washing on the surface of the application; this helps to extend the supply of drugs on the application site.
Colorants in Pharmaceutics



Colorants in PharmaceuticsLovnish Thakur
Ěý
Colorants or coloring agents are mainly used to impart a distinctive appearance to the pharmaceutical dosage forms.
We can also say that the colorants are the cosmetics for the pharmaceutical preparations, because the aesthetic appearance of dosage forms can be enhanced by using suitable colorants.
emulsion



emulsionsara27sara
Ěý
The document discusses different types of emulsions. It begins by defining an emulsion as a mixture of two or more immiscible liquids. It then describes four main types of emulsions: oil-in-water emulsions, water-in-oil emulsions, multiple emulsions (O/W/O and W/O/W), and microemulsions. The key differences between O/W and W/O emulsions are also summarized. Detection tests for identifying the type of emulsion are then outlined.emulsion



emulsionsara27sara
Ěý
The document discusses emulsions, which are mixtures of two or more liquids that do not normally mix. It defines the key types of emulsions as oil-in-water (O/W), water-in-oil (W/O), and multiple emulsions. It also explains the differences between O/W and W/O emulsions and describes detection tests that can identify the emulsion type. Finally, it provides examples of common emulsifying agents like lecithin, soap, and gum and discusses their properties and uses in emulsions.More Related Content
What's hot (20)
Elixirs



ElixirsSadaqat Ali
Ěý
Elixirs are clear, sweetened alcoholic solutions intended for oral use. They contain 10-12% alcohol which helps dissolve ingredients. Elixirs differ from syrups in that alcohol is always present in elixirs and they remain clear while syrups can contain dyes. Common types of elixirs include simple non-medicated elixirs and medicated elixirs containing active ingredients. Elixirs are prepared by separately dissolving water and alcohol soluble components before combining the solutions and adding excipients like sweeteners, flavors, and preservatives.Pharmaceutics - emulsions



Pharmaceutics - emulsionsAreej Abu Hanieh
Ěý
An emulsion is an unstable mixture of two immiscible liquids, where one liquid is dispersed as globules in the other liquid. Emulsions can be oil-in-water or water-in-oil depending on the continuous and dispersed phases. Surfactants are needed to stabilize emulsions by lowering surface tension at the interface between the liquids. The document discusses different types of emulsifiers including surface-active agents, hydrocolloids, and solid particles that stabilize emulsions through monomolecular or multimolecular film formation. It also covers emulsion characterization, applications in pharmaceutical products, and factors affecting emulsion stability.Pharmaceutical Suspensions and Emulsions



Pharmaceutical Suspensions and EmulsionsPallavi Kurra
Ěý
A detailed description about Pharmaceutical Suspensions and Emulsions or Disperse systems was given in this pptSuspensions



SuspensionsSaif Khan
Ěý
The document discusses pharmaceutical suspensions. A suspension is a coarse dispersion where an insoluble internal phase is dispersed uniformly throughout an external phase. Reasons for formulating suspensions include insolubility of the drug, masking bitter taste, increasing stability, and achieving controlled drug release. Common types of suspensions include antacids, antibiotics, analgesics, anthelmintics, and antifungals. Suspending agents are used to prevent sedimentation and ensure uniform dosing. Preparation involves grinding the insoluble drug into a paste and incorporating suspending agents before making up the final volume. Advantages include improved stability and bioavailability for some drugs, while disadvantages include issues with physical stability and accurate dosing.Monophasic liquid dosage forms part 1 



Monophasic liquid dosage forms part 1 Prof. Sandhya Lanke/ Sudrik
Ěý
Monophasic liquid dosage forms are liquid preparations containing two or more components in a single phase system that form a true solution. They have advantages like being easier to swallow, increasing drug absorption rates, and allowing flexible dosing. However, they can also be unstable, have unpleasant tastes, and require accuracy in dosing. The document further classifies liquid dosage forms and discusses mixtures, syrups, elixirs, and other types used internally or externally.Semisolid dosage -Ointments, Pate,Jellies



Semisolid dosage -Ointments, Pate,JelliesRavikumar Patil
Ěý
This document provides information about semisolid dosage forms such as ointments, pastes, and jellies. It defines semisolids as topical dosage forms used for therapeutic, protective, or cosmetic purposes. The key ingredients in semisolids include a base, preservatives, humectants, antioxidants, emulsifiers, gelling agents, permeation enhancers, and buffers. The document discusses the ideal properties of bases and lists common bases such as petrolatum, lanolin, and polyethylene glycol. It also covers the advantages and disadvantages of semisolid dosage forms.Emusification



Emusificationhassanraza406
Ěý
The document defines an emulsion as a mixture of two or more liquids that are normally immiscible. It then discusses the internal and external phases of emulsions, types of emulsions based on dispersed phase and size, advantages and disadvantages, identification tests, emulsifying agents, theories of emulsification, and factors that contribute to emulsion stability like interfacial tension. In summary, the key topics covered are the definition of emulsions, classification based on phase and size, tests to identify emulsion types, role of emulsifying agents, and theories to explain emulsion stabilization mechanisms.LIQUID DOSAGE FORMS.pptx



LIQUID DOSAGE FORMS.pptxSUJITHA MARY
Ěý
Liquid dosage forms: Advantages and disadvantages of liquid dosage forms. Excipients used in formulation of liquid dosage forms. Solubility enhancement techniquesInstability Of Emulsions.pptx



Instability Of Emulsions.pptxYashThorat20
Ěý
The document discusses various types of instability that can occur in pharmaceutical emulsions, including flocculation, coalescence, creaming, breaking, deterioration by microorganisms, and physical/chemical changes. Flocculation involves droplets aggregating into larger units while coalescence occurs when droplets merge to form even larger droplets. Creaming is the rising of dispersed globules, and breaking refers to the complete separation of emulsion phases. Proper formulation, storage conditions, and addition of preservatives can help prevent these instability issues.Monophasic liquid dosage forms part 3



Monophasic liquid dosage forms part 3Prof. Sandhya Lanke/ Sudrik
Ěý
In this presentation viewers will able to learn about liquids for internal administration such as syrups, elixirs and linctus.Suspending agents



Suspending agentsSILVI SINGH
Ěý
This document discusses different types of suspending agents used in pharmaceutical formulations. It classifies suspending agents into polysaccharides, inorganic salts, and synthetic compounds. Some examples of polysaccharides agents include acacia, tragacanth, and starches. Common inorganic salts are bentonite, aluminum magnesium silicate, and aluminum hydroxide. Synthetic agents include carbomers and colloidal silicon dioxide. Suspending agents help stabilize suspensions by increasing viscosity and slowing particle sedimentation according to Stokes' law. They prevent caking and can be resuspended with agitation.Monophasic liquid dosage forms part 4



Monophasic liquid dosage forms part 4Prof. Sandhya Lanke/ Sudrik
Ěý
In this presentation viewers will able to learn about liquids for external use such as liniments and lotions, liquids for oral cavity such as mouthwash, throat paints and gargles.Test for identification of type of emulsion



Test for identification of type of emulsionSantuMistree4
Ěý
Four tests are used to identify oil-in-water (O/W) and water-in-oil (W/O) emulsions: the dilution test, dye test, conductivity test, and fluorescence test. The dilution test identifies the emulsion type based on whether it dilutes easily with water or oil. The dye test observes emulsion droplets under a microscope after adding an oil-soluble dye. If the continuous phase is colored and droplets are clear, it is a W/O emulsion; if droplets are colored and the continuous phase is clear, it is an O/W emulsion. The conductivity test uses electrodes - if a bulb glows, it is an O/W emulsion, and if not, it is aStability of suspensions



Stability of suspensionsAbdelrhman abooda
Ěý
This document discusses stability factors and applications of pharmaceutical suspensions. It notes that small particle size, increasing viscosity, and maintaining optimal temperature contribute to suspension stability. Suspensions are used for insoluble drugs, to improve drug stability, and to mask unpleasant tastes. Key factors for stability include particle size, viscosity, temperature, surfactants, hydrophilic colloids, solvents, and proper mixing procedures.Emulsions



EmulsionsIqra Zulfiqar Ali Rajput
Ěý
This document provides an overview of pharmaceutical emulsions. It defines emulsions as dispersions of one liquid in another immiscible liquid, stabilized by an emulsifying agent. The key topics covered include the classification of emulsions as oil-in-water or water-in-oil, theories of emulsification, common emulsifying agents like surfactants and hydrocolloids, and factors affecting the stability of emulsions such as flocculation and creaming. Pharmaceutical applications of emulsions include lotions, creams, and ointments.Monophasic liquid dosage form ppt



Monophasic liquid dosage form pptmeenakharwade1
Ěý
This document provides information on various monophasic liquid dosage forms including gargles, mouthwashes, throat paints, and syrups. It discusses the components, advantages, disadvantages, and methods of preparation for each type. Gargles are aqueous solutions used to treat throat infections that are prepared by dissolving ingredients in solvents. Mouthwashes are solutions used for oral hygiene that can be cosmetic or therapeutic. Throat paints are viscous liquids applied to the mouth and throat to treat infections. Syrups are concentrated sugar solutions that can also contain medication, providing a pleasant way to administer liquid drugs. The document outlines the typical ingredients and formulations for each monophasic liquid dosage form.Semisolid dosage forms: Paste and Jellies



Semisolid dosage forms: Paste and JelliesParag Jain
Ěý
Difference between ointments and paste, preparation of paste, preservatives, jellies, and preparations, poultices Semisolid dosage forms ppt



Semisolid dosage forms pptPranatiChavan
Ěý
Semisolid dosage forms are neither solid nor liquid, however, they are a combination or mixture of both, and they used for both local and systemic effects. Pharmaceutical semisolid dosage forms such as creams, ointments, gels, suppositories, and paste are used for topical application. Semisolid dosage forms are intended used as drug carriers that are transported topically through the skin, buckle tissue, rectal tissue, outer ear lining nasal mucosa, urethral membrane, vagina, and cornea. The semisolid may adhere adequately before washing on the surface of the application; this helps to extend the supply of drugs on the application site.
Colorants in Pharmaceutics



Colorants in PharmaceuticsLovnish Thakur
Ěý
Colorants or coloring agents are mainly used to impart a distinctive appearance to the pharmaceutical dosage forms.
We can also say that the colorants are the cosmetics for the pharmaceutical preparations, because the aesthetic appearance of dosage forms can be enhanced by using suitable colorants.
emulsion



emulsionsara27sara
Ěý
The document discusses different types of emulsions. It begins by defining an emulsion as a mixture of two or more immiscible liquids. It then describes four main types of emulsions: oil-in-water emulsions, water-in-oil emulsions, multiple emulsions (O/W/O and W/O/W), and microemulsions. The key differences between O/W and W/O emulsions are also summarized. Detection tests for identifying the type of emulsion are then outlined.Similar to Emulsions (20)
emulsion



emulsionsara27sara
Ěý
The document discusses emulsions, which are mixtures of two or more liquids that do not normally mix. It defines the key types of emulsions as oil-in-water (O/W), water-in-oil (W/O), and multiple emulsions. It also explains the differences between O/W and W/O emulsions and describes detection tests that can identify the emulsion type. Finally, it provides examples of common emulsifying agents like lecithin, soap, and gum and discusses their properties and uses in emulsions.Emulsions



EmulsionsSaif Khan
Ěý
An emulsion is an unstable mixture of two immiscible liquids stabilized by an emulsifying agent. The document defines emulsions and describes different types including oil-in-water, water-in-oil, multiple, and microemulsions. Methods for preparing emulsions like the continental, English, and bottle methods are outlined. Advantages of emulsions include masking unpleasant tastes, enabling oral or parenteral administration of insoluble compounds, and providing sustained release. However, emulsions are thermodynamically unstable and require proper formulation to avoid issues like creaming or cracking.EMULSION by deepak kumar Assistant Professor Of SHRI RAM COLLEGE OF PHARMA...



EMULSION by deepak kumar Assistant Professor Of SHRI RAM COLLEGE OF PHARMA...Deepak Kumar
Ěý
Emulsion in pharmaceutics - I,
B. pharm
upload by deepak kumar
Assistant Professor Of SHRI RAM COLLEGE OF PHARMACYEMULSION by deepak kumar Assistant Professor Of SHRI RAM COLLEGE OF PHARMA...



EMULSION by deepak kumar Assistant Professor Of SHRI RAM COLLEGE OF PHARMA...Deepak Kumar
Ěý
Emulsion in pharmaceutics - I,
B. pharm
upload by deepak kumar
Assistant Professor Of SHRI RAM COLLEGE OF PHARMACYEMULSIONS.pptx



EMULSIONS.pptxHizbaMemon
Ěý
An emulsion consists of two immiscible liquids, where one liquid is dispersed as fine droplets in the other. Emulsions can be oil-in-water or water-in-oil depending on which liquid is the continuous and dispersed phases. Multiple emulsions containing water and oil droplets are also possible. Emulsions are used orally, topically, and parenterally in pharmaceutical products. Stability is achieved through emulsifying agents which reduce interfacial tension between phases. The type of emulsion depends on the solubility of the emulsifying agent used.Emulsion.pptx



Emulsion.pptxMadhu B K
Ěý
This document provides information on emulsions, including:
- Emulsions are biphasic liquid preparations containing two immiscible liquids, one dispersed as globules in the other.
- The main types are oil-in-water and water-in-oil emulsions. Oil-in-water emulsions are generally preferred for internal use.
- Emulsions can be identified using dilution, dye, fluorescence, and conductivity tests.
- The stability of emulsions can be impacted by cracking, creaming, phase inversion, and other factors. Proper formulation and storage are important to maintain stability.7 biphasic liquid dosage form emulsion



7 biphasic liquid dosage form emulsionPradeep Patil
Ěý
An emulsion is a dispersion of one liquid into another immiscible liquid. Emulsions can be oil-in-water (O/W) or water-in-oil (W/O) depending on the dispersed and continuous phases. Emulsifiers form an interfacial film between the phases that stabilizes the emulsion. Pharmaceutically acceptable emulsifiers must be non-toxic, stable, and compatible with other ingredients. Emulsions are used for oral, topical, parenteral, and other routes of administration. Various natural, semi-synthetic, and synthetic agents can act as emulsifiers including acacia, gelatin, polysorbates, and soaps. Hydrophile-lipEmulsion - Physical Pharmacy



Emulsion - Physical PharmacyAdarshPatel73
Ěý
This document discusses emulsions, which are biphasic systems consisting of two immiscible liquids, one dispersed as droplets in the other. An emulsifying agent is needed to stabilize the system and prevent separation. There are two main types of emulsions: oil-in-water, where oil is the dispersed phase, and water-in-oil, where water is dispersed. Multiple emulsions contain emulsions dispersed within another liquid. Emulsions can be used to deliver drugs, vitamins, and actives to the body. The mechanisms by which emulsifying agents stabilize emulsions involve reducing interfacial tension, forming protective films at the oil-water interface, and imparting charges to globules.18. Emulsion.pdf



18. Emulsion.pdfZaidShahid11
Ěý
Emulsions are thermodynamically unstable systems consisting of two immiscible liquids, one dispersed as globules in the other. Emulsifying agents are needed to stabilize the droplets and prevent separation. Emulsions can be oil-in-water or water-in-oil depending on the emulsifying agent used. Pharmaceutical applications of emulsions include masking bitter tastes, sustained drug release, and use in intravenous products. Emulsion stability can be affected by factors like globule size, density differences, and viscosity. Quality control tests assess properties such as particle size, viscosity, and phase separation over time.Formulation of Emulsion



Formulation of Emulsionসারন দাস
Ěý
An emulsion is a dispersion of one liquid into another immiscible liquid. The key types are oil-in-water (O/W) and water-in-oil (W/O) emulsions. Emulsions have various pharmaceutical applications like masking unpleasant tastes and enhancing drug absorption. Emulsion stability and type depend on factors like the emulsifying agent used, its HLB value, and emulsion preparation method. Common tests are used to identify the emulsion type and stability must be ensured through proper preservation, packaging, and storage.Emulsion 1 & 2.pptx



Emulsion 1 & 2.pptxAbsarAhmed29
Ěý
An emulsion is a dispersion of one liquid (the dispersed phase) as globules within another liquid (the continuous phase) in which it is immiscible. Emulsions are thermodynamically unstable and require an emulsifying agent to stabilize the system. There are two main types of emulsions - oil-in-water (O/W) emulsions where oil is the dispersed phase and water the continuous phase, and water-in-oil (W/O) emulsions where water is the dispersed phase and oil the continuous phase. Emulsions can be prepared using various methods depending on the scale and ingredients, such as the continental/dry gum method, English/wet gum method, or bottle methodEmulsion 8th industry Lecture pharmacy.pptx



Emulsion 8th industry Lecture pharmacy.pptxBeee7
Ěý
aqueous one known as a direct emulsion. Stabilization of O/W emulsion is often performed with hydrophilic-hydrophobic particles. The hydrophilic end of the emulsifier molecule has an affinity for water, and the hydrophobic end is drawn to the fat/oil. Vigorously mixing the emulsifier with the water and oil creates a stable emulsion. For example, milk is oil in the water type of emulsion. In this mixture, fat globules are dispersed in the water.
Emulsion water in oil (W/O) is composed of an aqueous phase dispersed in the oil phase. A water-in-oil emulsion is much fattier than a direct emulsion. Margarine is a water-in-oil emulsion.
Other emulsions, such as oil in water in oil, or water in oil in water, exist as well. Blood is also an emulsion consisting of negatively charged colloidal particles, which are albuminoid substances.
Go to:
Issues of Concern
Emulsions are a sub-class of colloids, which are two-phase systems of matter.
Although the terms colloid and emulsion are sometimes used indistinctly, emulsion applies only when both dispersed, and continuous phases are liquids. A colloid is a mixture of a compound that is in a solid, liquid, or gas state and a liquid. The critical difference between a colloid and an emulsion is that colloid can form when any state of matter (solid, gas, or liquid) combine with a liquid. In contrast, the emulsion has two liquid components that are initially immiscible with each other.
Emulsions, as liquids, do not demonstrate a static internal structure. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of droplets. Industries use emulsifying agents, eg, surfactants, to maintain a static structure.[1]
Usually, the phase in which the surfactant exhibits the greatest solubility is the continuous phase. Thus, hydrophilic surfactants foster O/W emulsions, whereas lipophilic surfactants promote W/O emulsions.
Go to:
Clinical Significance
Emulsions are frequently used in pharmaceuticals, personal hygiene products, and cosmetics. These are usually oil and water emulsions, albeit dispersed. These emulsions are called creams, ointments, balms, pastes, films, or liquids, depending on their oil-to-water ratios, the addition of other additives, and their intended administration route. Emulsions allow the encapsulation of an active ingredient in the dispersed phase to protect it from degradation and preserve its activity in a sustained manner. They are used to make medications more palatable, to improve their effectiveness via dosage control of active ingredients, and to provide better aesthetics for topical drugs such as ointments.
Intravenous and parenteral emulsions may be used for nutritive therapy applications when a patient is unable to consume food or receive nutrition. Fat emulsions serve as dietary complements for patients who cannot get the required fat solely from their diet. The compound may be given asPharmaceutical emulsion



Pharmaceutical emulsionBanaras Hindu University
Ěý
Definition: An emulsion is a biphasic liquids dosages from in which two immiscible liquids, one of which is dispersed as finite globules in the other.
Recently uploaded (20)
Neurologic Manifestations of Infective Endocarditis.pptx



Neurologic Manifestations of Infective Endocarditis.pptxdribnibrahem164
Ěý
neurological complications of infective endocarditisOne Health Rabies Control in Indonesia_APCAT meeting May 2022.pptx



One Health Rabies Control in Indonesia_APCAT meeting May 2022.pptxWahid Husein
Ěý
What is FAO doing to support rabies control programmes in Indonesia using One Health approachCharacteristics and Criteria of Good Research.pptx



Characteristics and Criteria of Good Research.pptxDr. Binu Babu Nursing Lectures Incredibly Easy
Ěý
Characteristics and Criteria of Good ResearchAdvancements in IgA Nephropathy: Discovering the Potential of Complement Path...



Advancements in IgA Nephropathy: Discovering the Potential of Complement Path...PVI, PeerView Institute for Medical Education
Ěý
Co-Chairs and Presenters, Gerald Appel, MD, and Dana V. Rizk, MD, discuss kidney disease in this CME activity titled “Advancements in IgA Nephropathy: Discovering the Potential of Complement Pathway Therapies.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/48UHvVM. CME credit will be available until February 25, 2026.Acute & Chronic Inflammation, Chemical mediators in Inflammation and Wound he...



Acute & Chronic Inflammation, Chemical mediators in Inflammation and Wound he...Ganapathi Vankudoth
Ěý
A complete information of Inflammation, it includes types of Inflammation, purpose of Inflammation, pathogenesis of acute inflammation, chemical mediators in inflammation, types of chronic inflammation, wound healing and Inflammation in skin repair, phases of wound healing, factors influencing wound healing and types of wound healing.Retinal Disease in Emergency Medicine: Timely Recognition and Referral for Sp...



Retinal Disease in Emergency Medicine: Timely Recognition and Referral for Sp...PVI, PeerView Institute for Medical Education
Ěý
Co-Chairs, Robert M. Hughes, DO, and Christina Y. Weng, MD, MBA, prepared useful Practice Aids pertaining to retinal vein occlusion for this CME activity titled “Retinal Disease in Emergency Medicine: Timely Recognition and Referral for Specialty Care.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3NyN81S. CME credit will be available until March 3, 2026.Increased Clinical Trial Complexity | Dr. Ulana Rey | MindLumina



Increased Clinical Trial Complexity | Dr. Ulana Rey | MindLuminaUlana Rey PharmD
Ěý
Increased Clinical Trial Complexity. By Ulana Rey PharmD for MindLumina. Dr. Ulana Rey discusses how clinical trial complexity—endpoints, procedures, eligibility criteria, countries—has increased over a 20-year period.Macafem Reviews 2024 - Macafem for Menopause Symptoms



Macafem Reviews 2024 - Macafem for Menopause SymptomsMacafem Supplement
Ěý
At Macafem, we provide 100% natural support for women navigating menopause. For over 20 years, we've helped women manage symptoms, and in 2024, we're proud to share their heartfelt experiences.FAO's Support Rabies Control in Bali_Jul22.pptx



FAO's Support Rabies Control in Bali_Jul22.pptxWahid Husein
Ěý
What is FAO doing to support rabies control programmes in Bali, Indonesia, using One Health approach with mass dog vaccination and integrated bite case management as main strategieslegal Rights of individual, children and women.pptx



legal Rights of individual, children and women.pptxRishika Rawat
Ěý
A legal right is a claim or entitlement that is recognized and protected by the law. It can also refer to the power or privilege that the law grants to a person. Human rights include the right to life and liberty, freedom from slavery and torture, freedom of opinion and expression, the right to work and educationDigestive Powerhouses: Liver, Gallbladder, and Pancreas for Nursing Students



Digestive Powerhouses: Liver, Gallbladder, and Pancreas for Nursing StudentsViresh Mahajani
Ěý
This educational PowerPoint presentation is designed to equip GNM students with a solid understanding of the liver, pancreas, and gallbladder. It explores the anatomical structures, physiological processes, and clinical significance of these vital organs. Key topics include:
Liver functions: detoxification, metabolism, and bile synthesis.
Gallbladder: bile storage and release.
Pancreas: exocrine and endocrine functions, including digestive enzyme and hormone production. This presentation is ideal for GNM students seeking a clear and concise review of these important digestive system components."Renal Physiology - Regulation of GFR and RBF



Renal Physiology - Regulation of GFR and RBFMedicoseAcademics
Ěý
1. Explain the physiological control of glomerular filtration and renal blood flow
2. Describe the humoral and autoregulatory feedback mechanisms that mediate the autoregulation of renal plasma flow and glomerular filtration rate
The influence of birth companion in mother care and neonatal outcome



The influence of birth companion in mother care and neonatal outcomelksharma10797
Ěý
this content related to birth companionship, role of birth companion in care of mother and neonatal HUMAN SEXUALITY AND SEXUAL RESPONCE CYCLE



HUMAN SEXUALITY AND SEXUAL RESPONCE CYCLEdaminipatel37
Ěý
It is all about topic of obg for new semester students E Book Daniya Sanal.pdf#healthy books.com



E Book Daniya Sanal.pdf#healthy books.comDaniyaSanal
Ěý
good health for good life good heart for safe and secure life..the good quality of life will makes good and #haelthy vibes...IMMUNO-ONCOLOGY DESCOVERING THE IMPORTANCE OF CLINICAL IMUNOLOGY IN MEDICINE



IMMUNO-ONCOLOGY DESCOVERING THE IMPORTANCE OF CLINICAL IMUNOLOGY IN MEDICINERelianceNwosu
Ěý
This presentation emphasizes the role of immunodiagnostics and Immunotherapy. Evidence - Based Practice - Nursing Research



Evidence - Based Practice - Nursing ResearchDr. Binu Babu Nursing Lectures Incredibly Easy
Ěý
Evidence-Based Practice - Nursing ResearchRole of Artificial Intelligence in Clinical Microbiology.pptx



Role of Artificial Intelligence in Clinical Microbiology.pptxDr Punith Kumar
Ěý
Artificial Intelligence (AI) is revolutionizing clinical microbiology by enhancing diagnostic accuracy, automating workflows, and improving patient outcomes. This presentation explores the key applications of AI in microbial identification, antimicrobial resistance detection, and laboratory automation. Learn how machine learning, deep learning, and data-driven analytics are transforming the field, leading to faster and more efficient microbiological diagnostics. Whether you're a researcher, clinician, or healthcare professional, this presentation provides valuable insights into the future of AI in microbiology.Thyroid Disorders in Pregnancy | Causes, Risks & Management



Thyroid Disorders in Pregnancy | Causes, Risks & ManagementDr.Laxmi Agrawal Shrikhande
Ěý
Explore the impact of thyroid disorders in pregnancy, including causes, risks, diagnosis, and management strategies to ensure maternal and fetal health.Advancements in IgA Nephropathy: Discovering the Potential of Complement Path...



Advancements in IgA Nephropathy: Discovering the Potential of Complement Path...PVI, PeerView Institute for Medical Education
Ěý
Acute & Chronic Inflammation, Chemical mediators in Inflammation and Wound he...



Acute & Chronic Inflammation, Chemical mediators in Inflammation and Wound he...Ganapathi Vankudoth
Ěý
Retinal Disease in Emergency Medicine: Timely Recognition and Referral for Sp...



Retinal Disease in Emergency Medicine: Timely Recognition and Referral for Sp...PVI, PeerView Institute for Medical Education
Ěý
Emulsions
- 1. EMULSIONS
- 2. An emulsion is a mixture of two or more immiscible liquids DISPERSED PHASECOUNTINIOUS PHASE
- 4. Micro Emulsions:- They are the emulsions that contain globules having diameter less than 0.1 micrometer. Multiple emulsions:- Oil in water or water in oil emulsions are dispersed in another liquid medium.
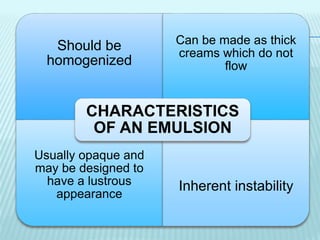
- 5. Should be homogenized Can be made as thick creams which do not flow Usually opaque and may be designed to have a lustrous appearance Inherent instability CHARACTERISTICS OF AN EMULSION
- 7. Continental or Dry Gum Method English or wet Gum Method Bottle or Forbes Bottle Method
- 8. EMULSIFYING AGENTS:- Be stable Be compatible with all other ingredients Be nontoxic Posses little odor, taste and color Not interfere with stability and efficacy of active agent. PHARMACEUTICALLY ACCEPTABLE EMULSIFIERS MUST ALSO
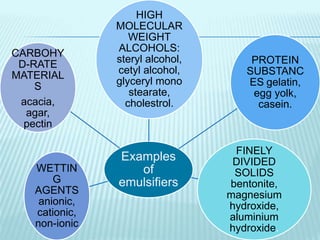
- 9. Examples of emulsifiers HIGH MOLECULAR WEIGHT ALCOHOLS: steryl alcohol, cetyl alcohol, glyceryl mono stearate, cholestrol. PROTEIN SUBSTANC ES gelatin, egg yolk, casein. FINELY DIVIDED SOLIDS bentonite, magnesium hydroxide, aluminium hydroxide. WETTIN G AGENTS anionic, cationic, non-ionic CARBOHY D-RATE MATERIAL S acacia, agar, pectin
- 10. IDENTIFICATION TESTS FOR EMULSIONS:-
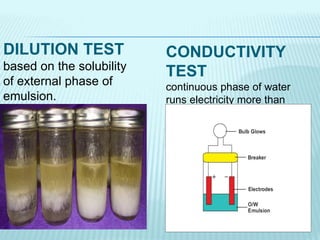
- 11. CONDUCTIVITY TEST continuous phase of water runs electricity more than continuous phase of oil. DILUTION TEST based on the solubility of external phase of emulsion.
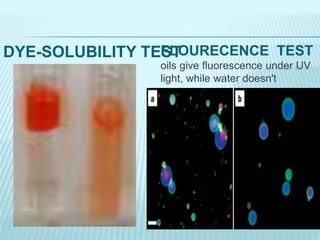
- 12. DYE-SOLUBILITY TESTFLOURECENCE TEST oils give fluorescence under UV light, while water doesn't
- 14. EMULSIONS are simple, have few Ingredients and are cheap. Reaction medium is mostly water therefore it easily penetrates in the skin. Beside these advantages following are certain uses of the emulsions.
- 15. Wide use in the food industry E .g. Vinaigrette, mayonnaise & milk Medicinal Use:- Emulsions that are more liquid may be used as oral medicine & cream based emulsions are best for topical application.
- 16. Used in beauty products e.g.. Creams and lotions. Used as polymerization e.g. paints and coatings.
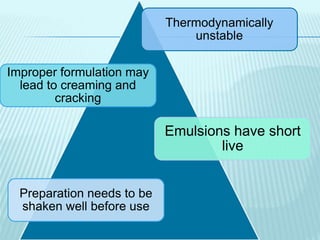
- 18. Thermodynamically unstable Improper formulation may lead to creaming and cracking Emulsions have short live Preparation needs to be shaken well before use
- 20. 1) Emulsion can be used to administer orally unpleasant tasting drugs 2) O/W type emulsion has been used for intravenous administration of oils oils and fats
- 21. 3) Bio availability of certain poorly soluble drugs can also be improved by dissolving them in oil and emulsifying 4)Emulsion of both O/W and W/O types has extensively been used to prepare pharmaceutical preparation for external use and cosmetic preparation such as screen and lotions
- 22. MADE BY :- SYEDA NEHAN IQBAL







