Equilibrium
Download as PPTX, PDF0 likes1,700 views
Equilibrium reactions can be categorized as fast, slow, or reversible reactions depending on their nature and experimental conditions. A reaction at equilibrium has reached a balance point where the rates of the forward and reverse reactions are equal. The position of equilibrium can be influenced by changing conditions like concentration, temperature, or pressure through Le Chatelier's principle.
1 of 91
Downloaded 18 times



























































































Recommended
Haloalkane and haloarene compounds



Haloalkane and haloarene compoundsSnehal Patel
Ěý
The document discusses the chemical reactions of haloalkanes and haloarenes. Haloalkanes undergo nucleophilic substitution reactions via SN1 and SN2 mechanisms, as well as elimination and reactions with metals like Grignard and Wrutz reactions. Haloarenes undergo nucleophilic substitution, electrophilic substitution, and reactions with metals. Nucleophilic substitution is the main reaction for both compounds.Alcohol, phenol and ether- Class 12 or Grade 12



Alcohol, phenol and ether- Class 12 or Grade 12Snehal Patel
Ěý
Alcohol, phenol and ether- Class 12, Grade 12,
Physical Properties, Preparations, Chemical Reactions Emulsion-class 12



Emulsion-class 12Snehal Patel
Ěý
Emulsion, Type of Emulsion, Test for identification of Emulsion, Demulsification.
Class 12,
Grade 12Hydrocarbons - Class 11



Hydrocarbons - Class 11Snehal Patel
Ěý
This document classifies and describes various types of hydrocarbons including alkanes, alkenes, alkynes, and benzene. It discusses their structures, methods of preparation from other compounds, and common chemical reactions. Key details provided include the IUPAC nomenclature rules for alkanes and cycloalkanes, reactions of alkenes with halogens, acids, and hydrogen, and benzene reactions such as nitration, sulfonation, halogenation, and Friedel-Crafts additions and acylations.Structure of atom



Structure of atomSnehal Patel
Ěý
Introduction
Discovery of Sub-atomic Particles
Atomic Models
Developments leading to Bohr’s Model of atom
Bohr’s Model for Hydrogen atom
Quantum Mechanical Model of the atoms
Electrochemistry



ElectrochemistrySnehal Patel
Ěý
Electrochemistry is a document prepared by Mr. Snehal Patel that discusses the Nernst equation and hydrogen fuel cells in 2 sections. It provides information on the Nernst equation, which describes the relationship between voltage and concentrations in electrochemical cells under non-standard-state conditions. It also covers hydrogen fuel cells and their use of hydrogen and oxygen to produce electricity through an electrochemical reaction.Solutions



SolutionsSnehal Patel
Ěý
This document discusses units of concentration used in chemistry solutions including molarity, molality, and normality. It also covers colligative properties such as vapor pressure, boiling point elevation, and freezing point depression. Finally, it mentions osmosis and the laws of osmotic pressure.Factors affecting drug degradation



Factors affecting drug degradationSnehal Patel
Ěý
PHYSICAL AND CHEMICAL DEGRADATION OF PHARMACEUTICAL PRODUCTS.
Physical Factors
Loss of volatile constituents
Loss of water
Absorption of water
Crystal growth
Polymorphism changes
Colour changes
Chemical factors
Hydrolysis
Oxidation
Carboxylation
Decarboxylation
Isomerization
Polymerization
Causes of Non linear pharmacokinetics



Causes of Non linear pharmacokineticsSnehal Patel
Ěý
Nonlinear pharmacokinetics can occur when the rate processes of drug absorption, distribution, metabolism, or excretion become dependent on dose size due to saturation of carrier proteins or enzymes. Some specific causes of nonlinearity include saturation of transporters during drug absorption, saturation of plasma protein binding sites or tissue binding sites during distribution, capacity-limited drug metabolism by enzyme saturation, and saturation of active tubular secretion or reabsorption processes during excretion. The Michaelis-Menten equation can describe the kinetics of these saturable, capacity-limited processes.Interfacial phenomena



Interfacial phenomenaSnehal Patel
Ěý
This document discusses interfacial phenomena such as surface tension, capillary rise, and wetting. It defines surface tension and explains how it is responsible for processes like droplet formation. It describes methods to determine surface and interfacial tension, including the capillary rise and Du Nouy ring methods. It also discusses concepts like surface free energy, spreading coefficients, work of cohesion/adhesion, and wetting phenomena. Wetting is important for processes like granulation, film coating, and dissolution. Surfactants can aid wetting by lowering interfacial tension and contact angles.Colloids



ColloidsSnehal Patel
Ěý
Definition
Application
Difference between molecular and Colloidal dispersion
Characteristics of dispersed phase
Classification of colloidal dispersion
Purification of colloidal dispersion
Environmental pollution



Environmental pollutionSnehal Patel
Ěý
This document discusses different types of environmental pollution including air, water, and land pollution. It defines pollution as undesirable changes that harm living things and ecosystems. The main causes of pollution are pollutants, which are substances produced by human activity that are present in greater than natural amounts and have detrimental effects. Pollutants are classified as degradable, slowly degradable, and non-degradable. Air pollution occurs when gases contaminate the air from sources like vehicles, industries, and power plants. Water pollution happens when harmful substances like sewage and chemicals pollute water bodies. Land becomes polluted when chemical and industrial wastes are discharged onto the surface.Ecosystem



EcosystemSnehal Patel
Ěý
This document defines key terms related to ecosystems, including producers, consumers, decomposers, food webs, populations and communities. It explains that an ecosystem is made up of both biotic (living) and abiotic (non-living) components that interact, including plants, animals, soil, air and water. Producers are organisms like plants that can produce their own food, while consumers eat other organisms and decomposers break down dead organic matter.Natural resources



Natural resourcesSnehal Patel
Ěý
Natural Resources
Renewable and non-renewable resources
Forest Resources
Water Resources
Mineral Resources
Food Resources
Energy Resources
Land Resources
Role of an individual in conservation of natural resources
Definition, scope and Importance of environment science



Definition, scope and Importance of environment scienceSnehal Patel
Ěý
Environmental science is a multidisciplinary field that combines various sciences to protect the environment. It studies both the biotic environment of living things like plants and animals, as well as the abiotic physical and chemical surroundings. The scope of environmental science is wide, as it examines how human activities impact natural resources in forests, rivers, cities, and more. Protecting the environment is important because natural resources are limited and human population growth is increasing resource usage and pollution, threatening human health and life itself if left unchecked. Individual action is needed alongside government efforts to preserve environmental resources for the future.Ophthalmic drug delivery systems



Ophthalmic drug delivery systemsSnehal Patel
Ěý
This document summarizes ophthalmic drug delivery systems. It discusses how absorption of eye drugs depends on physicochemical properties and tear drainage. Frequent dosing of short half-life drugs can cause irritation and side effects. One approach to improve effectiveness is prolonging drug contact with the cornea. The best known system is the ocular insert or ocusert, which provides controlled drug delivery over 7 days through rate-limiting membranes, in contrast to eye drops dosed 3-4 times daily.Intravaginal and intrauterine drug delivery system



Intravaginal and intrauterine drug delivery systemSnehal Patel
Ěý
This document summarizes intravaginal and intrauterine drug delivery systems. It discusses controlled release intravaginal systems used to deliver contraceptive steroids with advantages like no first pass effect and improved bioavailability. Two types of medicated intrauterine devices are described: copper IUDs, which consist of plastic with copper wire that releases ions for over 3 years of contraception, and progesterone IUDs, which are T-shaped devices that release 65 mcg of progesterone per day for 1 year of contraception.MICROBALLOONS: A NOVEL APPROACH IN GASTRO-RETENTION FLOATING DRUG DELIVERY SY...



MICROBALLOONS: A NOVEL APPROACH IN GASTRO-RETENTION FLOATING DRUG DELIVERY SY...Snehal Patel
Ěý
ABSTRACT
Oral controlled release dosage forms face several physiological restriction like inability to retain
and position the controlled drug delivery system within the targeted region of the gastrointestinal
tract (GIT) due to fluctuation in gastric emptying. This results in non�uniform absorption
pattern, inadequate medication release and shorter residence time of the dosage form in the
stomach. As the fallout of this episode there is inadequate absorption of the drug having
absorption window predominantly, in the upper area of GIT. These contemplations have
provoked to the development of oral controlled release dosage forms with gastroretentive
properties. Microballoons (Hollow microspheres) hold certification as one of the potential
approaches for gastric retention. Microballoons are spherical empty particles without core and
can remain in the gastric region for delayed periods. They significantly increase the gastric
residence time of medication, thereby enhance bioavailability, improves patient compliance by
reducing dosing frequency, lessen the medication waste, enhance retention of medication which
solubilize only in stomach, enhance solubility for medications that are less soluble at a higher pH
environment. The present review preparation methods, characterization, advantages,
disadvantages, mechanism of drug release from microballoons, applications and list of the drugs
formulated as microballoons are discussed.
KEYWORDS: Microballoons, Gastro-retention, Floating drug delivery system (FDDS).MICROSPONGE: A NOVEL APPROACH IN GASTRO-RETENTION DRUG DELIVERY SYSTEM (GRDDS)



MICROSPONGE: A NOVEL APPROACH IN GASTRO-RETENTION DRUG DELIVERY SYSTEM (GRDDS)Snehal Patel
Ěý
Oral controlled release dosage forms face several physiological restriction like inability to retain and position the controlled drug delivery system within the targeted region of the gastrointestinal tract (GIT) due to fluctuation in gastric emptying. This results in non‑uniform absorption pattern, inadequate medication release and shorter residence time of the dosage form in the stomach. As the fallout of this episode there is inadequate absorption of the drug having absorption window predominantly, in the upper area of GIT. These contemplations have provoked to the development of oral controlled release dosage forms with gastroretentive properties. Microsponge hold certification as one of the potential approaches for gastric retention. Microsponge are porous spherical empty particles without core and can remain in the gastric region for delayed periods. They significantly increase the gastric residence time of medication, thereby enhance bioavailability, improves patient compliance by reducing dosing frequency, lessen the medication waste, enhance retention of medication which solubilize only in stomach, enhance solubility for medications that are less soluble at a higher pH environment. In the present review method of preparation, characterization, advantages, disadvantages and applications of floating microsponge are discussed. Please citeDesign, Development, Evaluation and Optimization of Microballoons of Telmisartan



Design, Development, Evaluation and Optimization of Microballoons of TelmisartanSnehal Patel
Ěý
Abstract: In present study an attempt was made to prepare microballoons of
Telmisartan by emulsion solvent diffusion technique for sustained delivery by
using polymers like Ethyl cellulose to extend the drug release for about 12 hours in
the upper GIT, which may result in enhanced absorption and there by improved
bioavailability. Formulation optimization of Telmisartan loaded microballoons was
carried out by using different concentration of Polyvinyl alcohol (PVA) and Ethyl
cellulose. Total 9 batches were formulated. All 9 batches were evaluated for
entrapment efficiency (EE) and buoyancy. Among all batches DP4 shows
maximum entrapment efficiency (EE) and buoyancy and was considered as
optimized formulation. DP4 batch was further used for process optimization. The
process optimization was carried out at three different stirring speeds i.e. 1300,
1500 and 1700 rpm for three different stirring time period i.e. 1hr, 2hr and 3 hr and
another 9 batches were formulated. Out of all the batches DP13 showed the
spherical shape of microballoons without formation of flakes. Optimized batch
DP13 was evaluated for Zeta Potential, Particle Size Distribution which show -
41.8mV and 1.344 ÎĽm particle size, SEM, XRD Analysis. Batch DP13 was
charged for stability and were placed in glass vials container and stored at ICH
storage condition (2°C - 4°C Refrigeration condition , 30 ± 2°C / 60% ± 5% RH ,
40 ± 2°C / 75% ± 5% RH ) for a period of 30 days. The samples were analyzed for
physical appearance, buoyancy and for the drug release after 30 days. After 1
months samples were withdrawn and microballoons showed no change in physical
appearances, buoyancy and drug release, which indicate that the microballoons
were stable.
Keywords: Telmisartan, Microballoons, Emulsion solvent diffusion technique,
Buoyancy, Entrapment Efficiency.Introduction to microbiology



Introduction to microbiologySnehal Patel
Ěý
This document provides an introduction to microbiology. It defines microbiology as the study of microorganisms like bacteria, fungi, algae, protozoa and viruses. These microorganisms are too small to be seen with the naked eye and are present everywhere in the environment and in and on humans and other animals. Microorganisms can be beneficial or harmful. The document then outlines the main branches of microbiology like bacteriology, mycology, and parasitology. It also discusses several applied branches like medical microbiology, pharmaceutical microbiology, and food microbiology. Finally, it reviews some important figures in the history of microbiology like Louis Pasteur, Robert Koch, Joseph Lister and their contributions to the fieldProtein drug binding



Protein drug bindingSnehal Patel
Ěý
Protein binding of drugs can be reversible or irreversible. Reversible binding involves weak interactions like hydrogen bonds or hydrophobic bonds, while irreversible binding results from covalent bonds. Drugs bind to plasma proteins like albumin and alpha-1-acid glycoprotein, as well as to components in blood cells and extravascular tissues. The extent of protein binding affects the absorption, distribution, metabolism, and excretion of drugs. It determines the amount of active, unbound drug available to elicit its pharmacological response. Protein binding is influenced by factors related to the drug, binding proteins, and patient characteristics. It is important for understanding a drug's pharmacokinetics and pharmacodynamics.Interferon



InterferonSnehal Patel
Ěý
Interferons are proteins released by virus-infected cells that activate immune responses in nearby cells. There are three main types of interferons - alpha, beta, and gamma - which are produced by different immune cells. Interferons have general characteristics such as being heat-resistant glycoproteins with antiviral properties. They are used to treat various viral infections and cancers.Structure of virus



Structure of virusSnehal Patel
Ěý
Viruses lack cellular structures like nuclei, cytoplasm, and cell membranes. They contain nucleic acid surrounded by a protein coat called a capsid. Some viruses have an envelope outside the capsid made of lipids and proteins. Viruses' nucleic acids can be DNA or RNA, single or double stranded. Bacteriophages follow two life cycles: lytic, causing host cell lysis, or lysogenic, where the phage DNA integrates into the host chromosome without killing the cell.Microbiological assay



Microbiological assaySnehal Patel
Ěý
Two general methods are used for microbiological assays
Method A: Cylinder plate method or cup plate method.
Method B: Tube assay method or titrimetric method.
Sterilization



SterilizationSnehal Patel
Ěý
Introduction
Sterilization method
Equipment's involved in large scale sterilization
Sterilization indicators
Evaluation of efficiency of sterilization /Sterility testing
Design of aseptic area



Design of aseptic areaSnehal Patel
Ěý
The document discusses the design of aseptic areas for sterile product production. It outlines several key aspects of aseptic area design including:
- Dividing the production area into clean-up, compounding, aseptic, quarantine, and packaging areas.
- Using smooth, cleanable materials like stainless steel, glass or epoxy paint for floors, walls and ceilings.
- Fitting doors and windows flush to walls and maintaining positive air pressure and airflow.
- Supplying high-efficiency filtered air through HEPA filters and laminar airflow benches.
- Controlling airflow patterns to avoid particle generation and maintaining cleanroom classifications.Blind spots in AI and Formulation Science, IFPAC 2025.pdf



Blind spots in AI and Formulation Science, IFPAC 2025.pdfAjaz Hussain
Ěý
The intersection of AI and pharmaceutical formulation science highlights significant blind spots—systemic gaps in pharmaceutical development, regulatory oversight, quality assurance, and the ethical use of AI—that could jeopardize patient safety and undermine public trust. To move forward effectively, we must address these normalized blind spots, which may arise from outdated assumptions, errors, gaps in previous knowledge, and biases in language or regulatory inertia. This is essential to ensure that AI and formulation science are developed as tools for patient-centered and ethical healthcare.More Related Content
More from Snehal Patel (20)
Factors affecting drug degradation



Factors affecting drug degradationSnehal Patel
Ěý
PHYSICAL AND CHEMICAL DEGRADATION OF PHARMACEUTICAL PRODUCTS.
Physical Factors
Loss of volatile constituents
Loss of water
Absorption of water
Crystal growth
Polymorphism changes
Colour changes
Chemical factors
Hydrolysis
Oxidation
Carboxylation
Decarboxylation
Isomerization
Polymerization
Causes of Non linear pharmacokinetics



Causes of Non linear pharmacokineticsSnehal Patel
Ěý
Nonlinear pharmacokinetics can occur when the rate processes of drug absorption, distribution, metabolism, or excretion become dependent on dose size due to saturation of carrier proteins or enzymes. Some specific causes of nonlinearity include saturation of transporters during drug absorption, saturation of plasma protein binding sites or tissue binding sites during distribution, capacity-limited drug metabolism by enzyme saturation, and saturation of active tubular secretion or reabsorption processes during excretion. The Michaelis-Menten equation can describe the kinetics of these saturable, capacity-limited processes.Interfacial phenomena



Interfacial phenomenaSnehal Patel
Ěý
This document discusses interfacial phenomena such as surface tension, capillary rise, and wetting. It defines surface tension and explains how it is responsible for processes like droplet formation. It describes methods to determine surface and interfacial tension, including the capillary rise and Du Nouy ring methods. It also discusses concepts like surface free energy, spreading coefficients, work of cohesion/adhesion, and wetting phenomena. Wetting is important for processes like granulation, film coating, and dissolution. Surfactants can aid wetting by lowering interfacial tension and contact angles.Colloids



ColloidsSnehal Patel
Ěý
Definition
Application
Difference between molecular and Colloidal dispersion
Characteristics of dispersed phase
Classification of colloidal dispersion
Purification of colloidal dispersion
Environmental pollution



Environmental pollutionSnehal Patel
Ěý
This document discusses different types of environmental pollution including air, water, and land pollution. It defines pollution as undesirable changes that harm living things and ecosystems. The main causes of pollution are pollutants, which are substances produced by human activity that are present in greater than natural amounts and have detrimental effects. Pollutants are classified as degradable, slowly degradable, and non-degradable. Air pollution occurs when gases contaminate the air from sources like vehicles, industries, and power plants. Water pollution happens when harmful substances like sewage and chemicals pollute water bodies. Land becomes polluted when chemical and industrial wastes are discharged onto the surface.Ecosystem



EcosystemSnehal Patel
Ěý
This document defines key terms related to ecosystems, including producers, consumers, decomposers, food webs, populations and communities. It explains that an ecosystem is made up of both biotic (living) and abiotic (non-living) components that interact, including plants, animals, soil, air and water. Producers are organisms like plants that can produce their own food, while consumers eat other organisms and decomposers break down dead organic matter.Natural resources



Natural resourcesSnehal Patel
Ěý
Natural Resources
Renewable and non-renewable resources
Forest Resources
Water Resources
Mineral Resources
Food Resources
Energy Resources
Land Resources
Role of an individual in conservation of natural resources
Definition, scope and Importance of environment science



Definition, scope and Importance of environment scienceSnehal Patel
Ěý
Environmental science is a multidisciplinary field that combines various sciences to protect the environment. It studies both the biotic environment of living things like plants and animals, as well as the abiotic physical and chemical surroundings. The scope of environmental science is wide, as it examines how human activities impact natural resources in forests, rivers, cities, and more. Protecting the environment is important because natural resources are limited and human population growth is increasing resource usage and pollution, threatening human health and life itself if left unchecked. Individual action is needed alongside government efforts to preserve environmental resources for the future.Ophthalmic drug delivery systems



Ophthalmic drug delivery systemsSnehal Patel
Ěý
This document summarizes ophthalmic drug delivery systems. It discusses how absorption of eye drugs depends on physicochemical properties and tear drainage. Frequent dosing of short half-life drugs can cause irritation and side effects. One approach to improve effectiveness is prolonging drug contact with the cornea. The best known system is the ocular insert or ocusert, which provides controlled drug delivery over 7 days through rate-limiting membranes, in contrast to eye drops dosed 3-4 times daily.Intravaginal and intrauterine drug delivery system



Intravaginal and intrauterine drug delivery systemSnehal Patel
Ěý
This document summarizes intravaginal and intrauterine drug delivery systems. It discusses controlled release intravaginal systems used to deliver contraceptive steroids with advantages like no first pass effect and improved bioavailability. Two types of medicated intrauterine devices are described: copper IUDs, which consist of plastic with copper wire that releases ions for over 3 years of contraception, and progesterone IUDs, which are T-shaped devices that release 65 mcg of progesterone per day for 1 year of contraception.MICROBALLOONS: A NOVEL APPROACH IN GASTRO-RETENTION FLOATING DRUG DELIVERY SY...



MICROBALLOONS: A NOVEL APPROACH IN GASTRO-RETENTION FLOATING DRUG DELIVERY SY...Snehal Patel
Ěý
ABSTRACT
Oral controlled release dosage forms face several physiological restriction like inability to retain
and position the controlled drug delivery system within the targeted region of the gastrointestinal
tract (GIT) due to fluctuation in gastric emptying. This results in non�uniform absorption
pattern, inadequate medication release and shorter residence time of the dosage form in the
stomach. As the fallout of this episode there is inadequate absorption of the drug having
absorption window predominantly, in the upper area of GIT. These contemplations have
provoked to the development of oral controlled release dosage forms with gastroretentive
properties. Microballoons (Hollow microspheres) hold certification as one of the potential
approaches for gastric retention. Microballoons are spherical empty particles without core and
can remain in the gastric region for delayed periods. They significantly increase the gastric
residence time of medication, thereby enhance bioavailability, improves patient compliance by
reducing dosing frequency, lessen the medication waste, enhance retention of medication which
solubilize only in stomach, enhance solubility for medications that are less soluble at a higher pH
environment. The present review preparation methods, characterization, advantages,
disadvantages, mechanism of drug release from microballoons, applications and list of the drugs
formulated as microballoons are discussed.
KEYWORDS: Microballoons, Gastro-retention, Floating drug delivery system (FDDS).MICROSPONGE: A NOVEL APPROACH IN GASTRO-RETENTION DRUG DELIVERY SYSTEM (GRDDS)



MICROSPONGE: A NOVEL APPROACH IN GASTRO-RETENTION DRUG DELIVERY SYSTEM (GRDDS)Snehal Patel
Ěý
Oral controlled release dosage forms face several physiological restriction like inability to retain and position the controlled drug delivery system within the targeted region of the gastrointestinal tract (GIT) due to fluctuation in gastric emptying. This results in non‑uniform absorption pattern, inadequate medication release and shorter residence time of the dosage form in the stomach. As the fallout of this episode there is inadequate absorption of the drug having absorption window predominantly, in the upper area of GIT. These contemplations have provoked to the development of oral controlled release dosage forms with gastroretentive properties. Microsponge hold certification as one of the potential approaches for gastric retention. Microsponge are porous spherical empty particles without core and can remain in the gastric region for delayed periods. They significantly increase the gastric residence time of medication, thereby enhance bioavailability, improves patient compliance by reducing dosing frequency, lessen the medication waste, enhance retention of medication which solubilize only in stomach, enhance solubility for medications that are less soluble at a higher pH environment. In the present review method of preparation, characterization, advantages, disadvantages and applications of floating microsponge are discussed. Please citeDesign, Development, Evaluation and Optimization of Microballoons of Telmisartan



Design, Development, Evaluation and Optimization of Microballoons of TelmisartanSnehal Patel
Ěý
Abstract: In present study an attempt was made to prepare microballoons of
Telmisartan by emulsion solvent diffusion technique for sustained delivery by
using polymers like Ethyl cellulose to extend the drug release for about 12 hours in
the upper GIT, which may result in enhanced absorption and there by improved
bioavailability. Formulation optimization of Telmisartan loaded microballoons was
carried out by using different concentration of Polyvinyl alcohol (PVA) and Ethyl
cellulose. Total 9 batches were formulated. All 9 batches were evaluated for
entrapment efficiency (EE) and buoyancy. Among all batches DP4 shows
maximum entrapment efficiency (EE) and buoyancy and was considered as
optimized formulation. DP4 batch was further used for process optimization. The
process optimization was carried out at three different stirring speeds i.e. 1300,
1500 and 1700 rpm for three different stirring time period i.e. 1hr, 2hr and 3 hr and
another 9 batches were formulated. Out of all the batches DP13 showed the
spherical shape of microballoons without formation of flakes. Optimized batch
DP13 was evaluated for Zeta Potential, Particle Size Distribution which show -
41.8mV and 1.344 ÎĽm particle size, SEM, XRD Analysis. Batch DP13 was
charged for stability and were placed in glass vials container and stored at ICH
storage condition (2°C - 4°C Refrigeration condition , 30 ± 2°C / 60% ± 5% RH ,
40 ± 2°C / 75% ± 5% RH ) for a period of 30 days. The samples were analyzed for
physical appearance, buoyancy and for the drug release after 30 days. After 1
months samples were withdrawn and microballoons showed no change in physical
appearances, buoyancy and drug release, which indicate that the microballoons
were stable.
Keywords: Telmisartan, Microballoons, Emulsion solvent diffusion technique,
Buoyancy, Entrapment Efficiency.Introduction to microbiology



Introduction to microbiologySnehal Patel
Ěý
This document provides an introduction to microbiology. It defines microbiology as the study of microorganisms like bacteria, fungi, algae, protozoa and viruses. These microorganisms are too small to be seen with the naked eye and are present everywhere in the environment and in and on humans and other animals. Microorganisms can be beneficial or harmful. The document then outlines the main branches of microbiology like bacteriology, mycology, and parasitology. It also discusses several applied branches like medical microbiology, pharmaceutical microbiology, and food microbiology. Finally, it reviews some important figures in the history of microbiology like Louis Pasteur, Robert Koch, Joseph Lister and their contributions to the fieldProtein drug binding



Protein drug bindingSnehal Patel
Ěý
Protein binding of drugs can be reversible or irreversible. Reversible binding involves weak interactions like hydrogen bonds or hydrophobic bonds, while irreversible binding results from covalent bonds. Drugs bind to plasma proteins like albumin and alpha-1-acid glycoprotein, as well as to components in blood cells and extravascular tissues. The extent of protein binding affects the absorption, distribution, metabolism, and excretion of drugs. It determines the amount of active, unbound drug available to elicit its pharmacological response. Protein binding is influenced by factors related to the drug, binding proteins, and patient characteristics. It is important for understanding a drug's pharmacokinetics and pharmacodynamics.Interferon



InterferonSnehal Patel
Ěý
Interferons are proteins released by virus-infected cells that activate immune responses in nearby cells. There are three main types of interferons - alpha, beta, and gamma - which are produced by different immune cells. Interferons have general characteristics such as being heat-resistant glycoproteins with antiviral properties. They are used to treat various viral infections and cancers.Structure of virus



Structure of virusSnehal Patel
Ěý
Viruses lack cellular structures like nuclei, cytoplasm, and cell membranes. They contain nucleic acid surrounded by a protein coat called a capsid. Some viruses have an envelope outside the capsid made of lipids and proteins. Viruses' nucleic acids can be DNA or RNA, single or double stranded. Bacteriophages follow two life cycles: lytic, causing host cell lysis, or lysogenic, where the phage DNA integrates into the host chromosome without killing the cell.Microbiological assay



Microbiological assaySnehal Patel
Ěý
Two general methods are used for microbiological assays
Method A: Cylinder plate method or cup plate method.
Method B: Tube assay method or titrimetric method.
Sterilization



SterilizationSnehal Patel
Ěý
Introduction
Sterilization method
Equipment's involved in large scale sterilization
Sterilization indicators
Evaluation of efficiency of sterilization /Sterility testing
Design of aseptic area



Design of aseptic areaSnehal Patel
Ěý
The document discusses the design of aseptic areas for sterile product production. It outlines several key aspects of aseptic area design including:
- Dividing the production area into clean-up, compounding, aseptic, quarantine, and packaging areas.
- Using smooth, cleanable materials like stainless steel, glass or epoxy paint for floors, walls and ceilings.
- Fitting doors and windows flush to walls and maintaining positive air pressure and airflow.
- Supplying high-efficiency filtered air through HEPA filters and laminar airflow benches.
- Controlling airflow patterns to avoid particle generation and maintaining cleanroom classifications.Recently uploaded (20)
Blind spots in AI and Formulation Science, IFPAC 2025.pdf



Blind spots in AI and Formulation Science, IFPAC 2025.pdfAjaz Hussain
Ěý
The intersection of AI and pharmaceutical formulation science highlights significant blind spots—systemic gaps in pharmaceutical development, regulatory oversight, quality assurance, and the ethical use of AI—that could jeopardize patient safety and undermine public trust. To move forward effectively, we must address these normalized blind spots, which may arise from outdated assumptions, errors, gaps in previous knowledge, and biases in language or regulatory inertia. This is essential to ensure that AI and formulation science are developed as tools for patient-centered and ethical healthcare.BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAH



BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHcoacharyasetiyaki
Ěý
BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHRRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)



RRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)SONU HEETSON
Ěý
RRB ALP CBT 2 RAC Question Paper MCQ PDF Free Download. Railway Assistant Loco Pilot Mechanic Refrigeration and Air Conditioning Important Questions.Helping Autistic Girls Shine Webinar şÝşÝߣs



Helping Autistic Girls Shine Webinar şÝşÝߣsPooky Knightsmith
Ěý
For more information about my speaking and training work, visit: https://www.pookyknightsmith.com/speaking/Functional Muscle Testing of Facial Muscles.pdf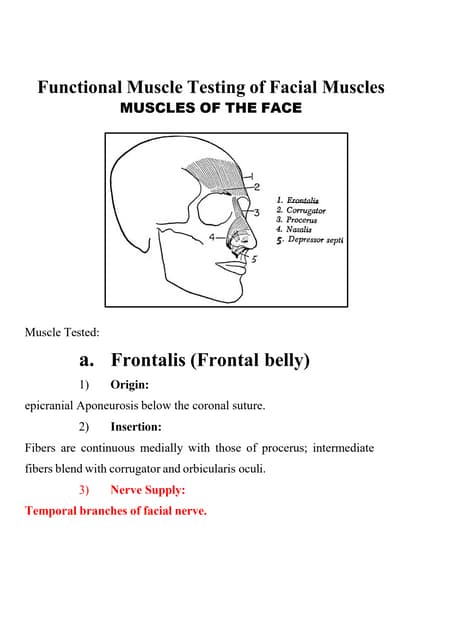



Functional Muscle Testing of Facial Muscles.pdfSamarHosni3
Ěý
Functional Muscle Testing of Facial Muscles.pdfInventory Reporting in Odoo 17 - Odoo 17 Inventory App



Inventory Reporting in Odoo 17 - Odoo 17 Inventory AppCeline George
Ěý
This slide will helps us to efficiently create detailed reports of different records defined in its modules, both analytical and quantitative, with Odoo 17 ERP.Chapter 2. Strategic Management: Corporate Governance.pdf



Chapter 2. Strategic Management: Corporate Governance.pdfRommel Regala
Ěý
This course provides students with a comprehensive understanding of strategic management principles, frameworks, and applications in business. It explores strategic planning, environmental analysis, corporate governance, business ethics, and sustainability. The course integrates Sustainable Development Goals (SDGs) to enhance global and ethical perspectives in decision-making.NUTRITIONAL ASSESSMENT AND EDUCATION - 5TH SEM.pdf



NUTRITIONAL ASSESSMENT AND EDUCATION - 5TH SEM.pdfDolisha Warbi
Ěý
NUTRITIONAL ASSESSMENT AND EDUCATION, Introduction, definition, types - macronutrient and micronutrient, food pyramid, meal planning, nutritional assessment of individual, family and community by using appropriate method, nutrition education, nutritional rehabilitation, nutritional deficiency disorder, law/policies regarding nutrition in India, food hygiene, food fortification, food handling and storage, food preservation, food preparation, food purchase, food consumption, food borne diseases, food poisoningAdministrative bodies( D and C Act, 1940



Administrative bodies( D and C Act, 1940P.N.DESHMUKH
Ěý
These presentation include information about administrative bodies such as D.T.A.B
CDL AND DCC, etc.Meeting the needs of modern students?, Selina McCoy



Meeting the needs of modern students?, Selina McCoyEconomic and Social Research Institute
Ěý
NAPD Annual Symposium
“Equity in our Schools: Does the system deliver for all young people?”ASP.NET Interview Questions PDF By ScholarHat



ASP.NET Interview Questions PDF By ScholarHatScholarhat
Ěý
ASP.NET Interview Questions PDF By ScholarHatOne Click RFQ Cancellation in Odoo 18 - Odoo şÝşÝߣs



One Click RFQ Cancellation in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide, we’ll discuss the one click RFQ Cancellation in odoo 18. One-Click RFQ Cancellation in Odoo 18 is a feature that allows users to quickly and easily cancel Request for Quotations (RFQs) with a single click.Full-Stack .NET Developer Interview Questions PDF By ScholarHat



Full-Stack .NET Developer Interview Questions PDF By ScholarHatScholarhat
Ěý
Full-Stack .NET Developer Interview Questions PDF By ScholarHatHow to create security group category in Odoo 17



How to create security group category in Odoo 17Celine George
Ěý
This slide will represent the creation of security group category in odoo 17. Security groups are essential for managing user access and permissions across different modules. Creating a security group category helps to organize related user groups and streamline permission settings within a specific module or functionality.Odoo 18 Accounting Access Rights - Odoo 18 şÝşÝߣs



Odoo 18 Accounting Access Rights - Odoo 18 şÝşÝߣsCeline George
Ěý
In this slide, we’ll discuss on accounting access rights in odoo 18. To ensure data security and maintain confidentiality, Odoo provides a robust access rights system that allows administrators to control who can access and modify accounting data. Equilibrium
- 1. Equilibrium
- 2. Introduction
- 6. • Depending on the nature of reaction and experimental condition, the reaction may be fast or slow. • Equilibrium reaction can be divided into following three categories:
- 43. x