Esters
- 3. KEY POINTS • Esters are a functional group commonly encountered in  organic chemistry. They are characterized by a carbon bound  to three other atoms: a single bond to a carbon, a double  bond to an oxygen, and a single bond to an oxygen. The singly  bound oxygen is bound to another carbon. • Ester names are derived from the parent alcohol and the  parent acid. While simple esters are often called by  their common names, all esters can be named using the  systematic IUPAC name, based on the name for the acid  followed by the suffix "-oate."
- 6. In the case of esters formed from common carboxylic  acids,  more  colloquial  terms  are  sometimes  used.  For  example,  ethanoic  acid  is  more  commonly  known  as  acetic  acid,  and  thus  its  esters  contain  "acetate"  instead  of  "ethanoate" in their names. Other such substitutions include  "formate" instead of "methanoate," "propionate" instead of  "propanoate,"  and  "butyrate"  instead  of  "butanoate."
- 7. Properties of Esters *Polar -Less than alcohols, carboxylic acids -Soluble in water *Smell Fruity *Can form Polymers Esters are more polar than ethers, but less so than alcohols. They  participate inhydrogen bonds as hydrogen bond acceptors, but cannot act  as hydrogen bond donors, unlike their parent alcohols and carboxylic  acids. This ability to participate in hydrogen bonding confers some water- solubility, depending on the length of the alkyl chains attached. Since they  have no hydrogens bonded to oxygens, as alcohols and carboxylic acids do,  esters do not self-associate. Consequently, esters are more volatile than  carboxylic acids of similar molecular weight.
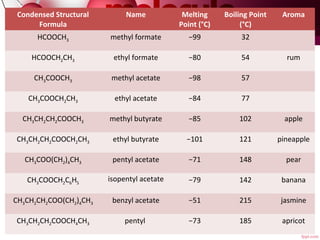
- 8. Condensed Structural Formula Name Melting Point (°C) Boiling Point (°C) Aroma HCOOCH3 methyl formate −99 32 HCOOCH2CH3 ethyl formate −80 54 rum CH3COOCH3 methyl acetate −98 57 CH3COOCH2CH3 ethyl acetate −84 77 CH3CH2CH2COOCH3 methyl butyrate −85 102 apple CH3CH2CH2COOCH2CH3 ethyl butyrate −101 121 pineapple CH3COO(CH2)4CH3 pentyl acetate −71 148 pear CH3COOCH2C6H5 isopentyl acetate −79 142 banana CH3CH2CH2COO(CH2)4CH3 benzyl acetate −51 215 jasmine CH3CH2CH2COOCH4CH3 pentyl  −73 185 apricot
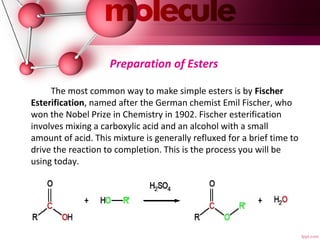
- 9. Preparation of Esters The most common way to make simple esters is by Fischer Esterification, named after the German chemist Emil Fischer, who won the Nobel Prize in Chemistry in 1902. Fischer esterification involves mixing a carboxylic acid and an alcohol with a small amount of acid. This mixture is generally refluxed for a brief time to drive the reaction to completion. This is the process you will be using today.
- 10. Esterification is the chemical process for making esters, which are compounds of the chemical structure R- COOR', where R and R' are either alkyl or aryl groups. The most common method for preparing esters is to heat a carboxylic acid, R-CO-OH, with an alcohol, R'-OH, while removing the water that is formed. A mineral acid catalyst is usually needed to make the reaction occur at a useful rate.
- 11. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated sulphuric acid. Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones where the carboxylic acid contains a benzene ring). Making esters from carboxylic acids
- 12. Saponification This is the process by which an ester is hydrolyzed in the presence of a base. The products of this reaction are the alcohol and the salt of the carboxylic acid used to create the ester. An example of this reaction is presented below:
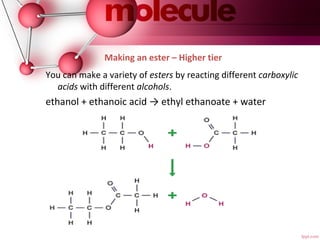
- 13. Making an ester – Higher tier You can make a variety of esters by reacting different carboxylic acids with different alcohols. ethanol + ethanoic acid → ethyl ethanoate + water
- 14. methanol + butanoic acid ‚Üí methyl butanoate + water A catalyst is needed to speed up the reaction. Concentrated sulfuric acid is often used.
- 15. Introduction to Esters’ Reactions • Esters can be converted into other esters (transesterification), the parent carboxylic acid (hydrolysis) or amides (see above). – Transesterification : heat with alcohol and acid catalyst – Hydrolysis : heat with aq. acid or base (e.g. aq. H2SO4 or aq. NaOH) (see hydrolysis of esters for more details) – Amide preparation : heat with the amine, methyl or ethyl esters are the most reactive Hydrolysis of Esters
- 16. Naming Esters Systematic names of esters are based on the name of the corresponding carboxylic acid. Remember esters look like this:
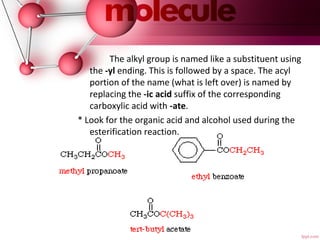
- 17. The alkyl group is named like a substituent using the -yl ending. This is followed by a space. The acyl portion of the name (what is left over) is named by replacing the -ic acid suffix of the corresponding carboxylic acid with -ate. * Look for the organic acid and alcohol used during the esterification reaction.
- 18. Example:
- 21. 1. methyl hexanoate 2. propyl pentanoate
- 22. Benzyl and Phenyl Benzyl - can be seen as a phenyl attached to a CH2 before attaching to the parent compound. Phenyl - the phenyl group or phenyl ring (often abbreviated as -Ph) is the functional group with the formula C6H5 - This functional group differs from the benzyl functional group in that benzyls have an extra CH2.
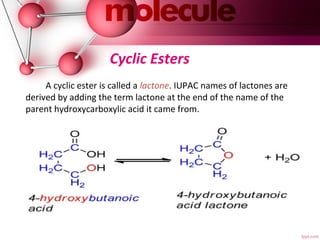
- 24. Cyclic Esters A cyclic ester is called a lactone. IUPAC names of lactones are derived by adding the term lactone at the end of the name of the parent hydroxycarboxylic acid it came from.
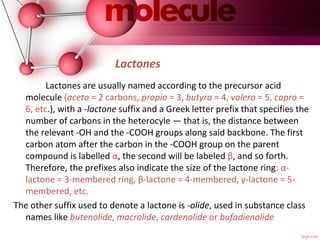
- 25. Lactones Lactones are usually named according to the precursor acid molecule (aceto = 2 carbons, propio = 3, butyro = 4, valero = 5, capro = 6, etc.), with a -lactone suffix and a Greek letter prefix that specifies the number of carbons in the heterocyle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α- lactone = 3-membered ring, β-lactone = 4-membered, γ-lactone = 5- membered, etc. The other suffix used to denote a lactone is -olide, used in substance class names like butenolide, macrolide, cardenolide or bufadienolide
- 26. Caprolactone
- 27. Butyrolactone
- 28. Propiolactone
- 29. Uses and Examples of Esters There are various uses of esters. 1. Esters that are have fragrant odors are used as a constituent of perfumes, essential oils, food flavorings, cosmetics, etc 2. Esters are used as an organic solvent 3. Natural esters are found in pheromones 4. Naturally occurring fats and oils are fatty acid esters of glycerol 5. Phospoesters form the backbone of DNA molecules 6. Nitrate esters, such as nitroglycerin, are known for their explosive properties 7. Polyesters are used to make plastics 8. Esters are used to make surfactants E.g. soap, detergents