Experiment 1
- 1. ESM 3024 Lab: Experiment #1 Measurements of Fluid Properties ÔÇô Density and Viscosity Authors: Drew Lovell John Naessens Date: 9/9/14 Time: 2:00 ÔÇô 4:50 p.m.
- 2. Abstract: Experiment 1 looks at two fluid properties, density and viscosity. Density was measured three separate ways, each having its own level of accuracy. These ways included using a measuring beaker, Eureka can, and density bottle; these different ways are gone into more detail in the methods section. Viscosity of these specimens was then found by using a falling sphere viscometer, which will also be explained in the methods section. The reason this experiment was conducted is that all fluids experience forces which cause motion throughout the flow. Understanding how fluids work and react to forces is important in many areas of life and across many fields. The results that were found displayed these characteristics and showed how density and viscosity can be calculated through a variety of procedures. Introduction: This experiment was performed to see how fluids are affected by their density and viscosity. Density is the measure of mass per unit volume and is denoted by the symbol, ¤ü. This measurement allows NewtonÔÇÖs Second Law to be applied to fluids on a ÔÇ£per unit volumeÔÇØ basis. The mass of the object is generally measured by a balance or scale, as where volume can be measured by water displacement, or by simply by calculating it using the objects parameters. Viscosity on the other hand is the measure of a fluids resistance to deformation. The more viscous a fluid is, the more resistance or thickness it will display. Viscosity is found through an equation which utilizes the objectÔÇÖs radius, densities of both the fluid and object suspended, and gravitational force. Both of these fluid properties can be seen in daily life since everything has density and every fluid has a viscosity. Motivation: The reason for completing this experiment was to learn how to calculate these fluid properties and to gain a better knowledge on measuring them accurately. Knowing how density and viscosity work is important for flotation, mixing of materials, fluid dynamics, and many other real world cases one might come into contact with. When measuring densities, the error of the experimentÔÇÖs calculations compared to actual values shows which method of taking density is more reliable. And when using the falling sphere viscometer it was also seen how accurate the gathered values were in comparison to actual known values. Comparing the data in this way indicates how close one can get to the actual values and how calculating viscosity and density is necessary for other practices.
- 3. Methods: 1.1.1 Density of Water using Measuring Beaker Density of water was calculated using a measuring beaker. Density is mass over volume. The mass of water was determined by using a scale and finding the difference in the mass of the beaker and the mass of a beaker with a designated volume of water. a) Measure mass of beaker b) Fill beaker with water, record volume c) Measure mass of beaker and water d) Calculate mass H2O e) Calculate density (Mass/Volume) f) Record possible error sources 1.1.2 Density of Water using Eureka Can Density of water was calculated using a Eureka can. A Eureka can is a metal can with a spout coming out the side. The can was filled with water until the water came out of the spout. The volume of three solid spheres was calculated by measuring their diameter and using the volume equation for a sphere. The volume of the spheres was the volume of water displaced from the Eureka can. The spheres were dropped into the Eureka can and the displaced water was collected in a beaker. The mass of water was determined by using a scale and finding the difference in the mass of the beaker and the mass of a beaker with a designated volume of water. The density was calculated using mass/volume. a) Calculate volume of three solid spheres provided b) Fill eureka can until water flows through spout, wait for flow to stop. c) Measure mass of beaker d) Place beaker under eureka can spout and place three spheres into eureka can. Allow beaker to collect water overflowing out of the spout and wait for the flow to stop. e) Measure mass of beaker and collected water. f) Calculate mass H2O g) Calculate density Mass/Volume h) Record possible error sources
- 4. 1.1.3 Density of Water using Density Bottle Density of water was calculated using a density bottle. The mass of the dried density bottle was measured using a scale. The bottle was filled with water and the outside was dried. The mass of the density bottle and water was measured using a scale. The mass of water was determined by finding the difference in the mass of the density bottle and the mass of the density bottle filled with water. The volume of the density bottle was 25ml. The density of the water was calculated using mass/volume. a) Obtain 25 ml bottle and stopper, measure mass b) Fill density bottle with water until it overflows, place stopper on top c) Dry outside of density bottle d) Measure mass of density bottle with water e) Calculate mass H2O f) Calculate density Mass/Volume g) Record possible error sources 1.1.4 Specific Weight of Glycerin and Castor Oil using a Hydrometer The specific weight of gylcerin and castor oil was calculated by using a hydrometer. A hydrometer was placed into each fluid to record the specific gravity. The specific gravity was used to calculate the density by multiplying the specific gravity by the density of water. The specific weight was calculated by multiplying the specific gravity with the specific weight of water. Calculated specific weight was compared to known specific weight to obtain percent error. a) Obtain two tall glass cylinders, one filled with castor oil and the other filled with glycerin b) Insert hydrometer into each fluid without contacting glass cylinder walls and wait for it to settle c) Record hydrometer level at bottom of meniscus d) Calculate density, specific weight, error
- 5. 1.1.5 Data Analysis The three values for density of water obtained in methods 1.1.1-3 were compared to the standard density of water. The most accurate method was determined and explained. Specific weights of glycerin and castor oil were compared to standard specific weights. Percentage error was explained. 1.3.1 Viscosity using falling spheres The viscosity of castor oil and glycerin were obtained using falling spheres. Spheres of three different sizes were individually dropped into each fluid. The distance they traveled through the fluid and the time it took to travel that distance was recorded. Each sized sphere was dropped three times in each fluid and the values were averaged. The velocities were calculated and used to determine the dynamic and kinematic velocities. a) Measure the mass of 10 spheres of each size and record, calculate mass of a single sphere and record. Calculate the volume of each sized sphere and record. b) Measure distance between timing bands on each cylinder (castor oil and glycerin) and record c) Drop each sized ball three times in each fluid. For every drop record the time it takes the ball to drop from one timing band to the other d) Calculate viscocity, dynamic and kinematic Results & Discussions 1.1.1 Density of Water using Measuring Beaker See Table 1.2 in appendix for data Mass of water = Mass of Beaker and Water ÔÇô Mass of Empty Beaker Density of water = Mass Water / Volume Water * 103 Density of water = 963.83 kg/m3 1.1.2 Density of Water using Eureka Can See table 1.3 in appendix for data Volume of sphere = 4/3*¤Ç*r3 Mass of Displaced Water = Mass of Beaker and Water ÔÇô Mass of Empty Beaker Density of water = Mass Water / Volume Water * 103 Density of water = 938.06 kg/m3 1.1.3 Density of Water using Density Bottle
- 6. See table 1.4 in appendix for data Mass of water = Mass of Bottle and Water ÔÇô Mass of Empty Bottle Density of water = Mass Water / Volume Water * 103 Density of water = 988.08 kg/m3 1.1.4 Specific Weight of Glycerin and Castor Oil using a Hydrometer See table 1.5 in appendix for data Density Calculated = Specific Gravity * 1000 kg/m3 Specific Weight Calculated = Specific Gravity * 981 N/m3 % error = abs((Actual ÔÇô Calculated)/Actual) Specific Weight Glycerin Calculated = 9466.65 %Error = .521 Specific Weight Castor Oil Calculated = 12360.6 %Error = .318 Error due to accuracy of meniscus reading 1.1.5 Data Analysis The most accurate density achieved was the density of water using the density bottle. The true density of water is 1000 kg/m3. The second most accurate density achieved was the density of water using a measuring beaker. The least accurate density achieved was the density of water using a Eureka can. The most accurate density was the density bottle because the density bottle is built to have an exact volume of water, removing human error in reading a meniscus, which is done using the measuring beaker of Eureka can. 1.3.1 Viscosity using falling spheres See Table 1.6 in Appendix for Sphere density data Volume of sphere = 4/3*¤Ç*r3 Density of Sphere = Mass of one sphere / Volume * 102 See Table 1.7 in appendix for Viscosity of Glycerin data, 1.8 for Castor Oil Velocity = Distance/Time ╬╝ = 2/(9u)*r2*(9.81)(¤üs- ¤üf) ¤üf = 1126.1 kg/m3 ╬¢ = ╬╝/¤ü Re = ¤üUD/╬╝ ╬╝ calculated Glycerin = 1.00, 1.08, 1.08 % error = 32.9, 27.5, 27.5 ╬╝ calculated Castor Oil = .891, .961, .966 % error = 9.6, 9.5, 9.5 Glycerin held a high percentage error. This was due to human error in recording the time it took to travel through the time bands and a low level of glycerin (there was no more glycerin to raise the level). The low level of glycerin caused the spheres to drop past the first time band very quickly and prevent an accurate starting time. The percentage error of Castor oil was relatively low compared to that of Glycerin.
- 7. Conclusion The density of water was calculated using a measuring beaker, eureka can, and density bottle. The density bottle was the most accurate way to measure the density of the water with a density of 988.08 kg/m3. The density bottle accuracy was followed by the measuring beaker then eureka can, which had error due to reading the meniscus. The specific weight of glycerin and castor oil were measured using a hydrometer. The hydrometer was very accurate with a percentage of error for glycerin of .521% and .318% for castor oil. The specific weight values obtained were 9467 (unitless) for castor oil and 12361 (unitless) for glycerin. The viscosities of glycerin and castor oil were measured using falling spheres. The viscosity of glycerin had a high average percentage error of 29.3% due to a lack of glycerin, which caused a misreading of the time it took to travel from time band to time band. Castor oil had a low average percentage error, relative to glycerin, at 9.5%. The values for viscosities and ReynoldÔÇÖs numbers can be seen in tables 1.7 and 1.8 of the appendix.
- 8. References: i. For Accepted Standard Values A. www.simmetric.co.uk/si_liquids.htm B. www.science.uwaterloo.ca/~cchieh/caet/c123/liquid.htm ii. Virginia Tech ESM 3024 Experiment 1 Lab Manual iii. Pictures A. Eureka Can http://www.andersonscientific.co.uk/images/medium/6165-01.jpg B. Density Bottle http://www.mrs-scientific. com/images/products/pics/WJ76.jpg C. Hydrometer http://avogadro-lab-supply. com/images/eBay/Hydrometer%20Reading.JPG D. Falling Sphere http://upload.wikimedia.org/wikipedia/commons/7/7b/Falling_Sphe re_Viscometer.gif
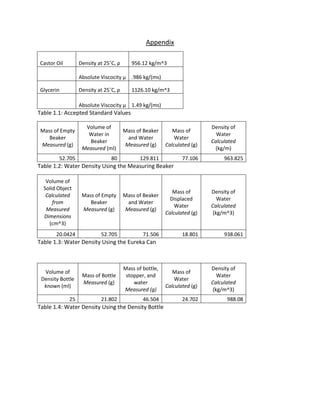
- 9. Appendix Castor Oil Density at 25╦ÜC, ¤ü 956.12 kg/m^3 Absolute Viscocity ╬╝ .986 kg/(ms) Glycerin Density at 25╦ÜC, ¤ü 1126.10 kg/m^3 Absolute Viscocity ╬╝ 1.49 kg/(ms) Table 1.1: Accepted Standard Values Mass of Empty Beaker Measured (g) Volume of Water in Beaker Measured (ml) Mass of Beaker and Water Measured (g) Mass of Water Calculated (g) Density of Water Calculated (kg/m) 52.705 80 129.811 77.106 963.825 Table 1.2: Water Density Using the Measuring Beaker Volume of Solid Object Calculated from Measured Dimensions (cm^3) Mass of Empty Beaker Measured (g) Mass of Beaker and Water Measured (g) Mass of Displaced Water Calculated (g) Density of Water Calculated (kg/m^3) 20.0424 52.705 71.506 18.801 938.061 Table 1.3: Water Density Using the Eureka Can Volume of Density Bottle known (ml) Mass of Bottle Measured (g) Mass of bottle, stopper, and water Measured (g) Mass of Water Calculated (g) Density of Water Calculated (kg/m^3) 25 21.802 46.504 24.702 988.08 Table 1.4: Water Density Using the Density Bottle
- 10. Liquid Specific Gravity Measured (unit-less) Density Calculated (kg/m^3) Specific Weight Calculated (N/m^3) Standard Specific Weight Known (N/m^3) % Error Between Stand and Calculated S.W. Calculated Castor Oil 0.965 965 9466.65 9417.6 0.521 Glycerin 1.26 1260 12360.6 12400 0.318 Table 1.5: Specific Weights of Glycerin and Castor Oil Diameter Measured (cm^3) Volume Calculated (cm^3) Number of Spheres Massed Total Mass of all Spheres Measured (g) Mass of one Sphere Calculated (g) Density of Spheres Calculated (kg/m^3) 0.199 0.00413 10 0.3 0.03 7270.49 0.299 0.0139 10 1.1 0.11 7859.24 0.399 0.0333 10 2.6 0.26 7817.29 Table 1.6: Data Sheet for Sphere Density Diameter Measured (cm) Time i (s) Time ii (s) Time iii (s) Table 1.7: Viscosity of Glycerin Dist. Sphere Falls (mm) Table 1.8: Viscosity of Castor Oil Vel I (m/s) Vel ii (m/s) Vel iii (m/s) Mean Vel (m/s) μ (Ns/ m^2) Λ (m^2/s) Re Calc'd Stand ard μ (Ns/ m^2) % Error for μ 0.199 18.06 18.4 18.47 242 0.0134 0.0132 0.0131 0.013 1.00 8.88*10^-4 0.0296 1.49 32.9 0.299 7.93 7.97 7.94 242 0.0305 0.0304 0.0305 0.031 1.08 9.59*10^-4 0.0951 1.49 27.5 0.399 4.53 4.47 4.47 242 0.0534 0.0541 0.0541 0.054 1.08 9.59*10^-4 0.2240 1.49 27.5 Diameter Measured (cm) Time i (s) Time ii (s) Time iii (s) Dist. Sphere Falls (mm) Vel I (m/s) Vel ii (m/s) Vel iii (m/s) Mean Vel (m/s) μ (Ns/ m^2) Λ (m^2/s) Re Calc'd Stand ard μ (Ns/ m^2) % Error for μ 0.199 15.75 16.13 16.06 250 0.0150 0.015 0.016 0.015 0.89 9.32*10^-4 0.0327 0.986 9.6 0.299 7.93 7.97 7.94 242 0.0305 0.0304 0.0305 0.031 1.08 1.01*10^-3 0.0807 0.986 9.5 0.399 4.53 4.47 4.47 242 0.0534 0.0541 0.0541 0.054 1.08 1.01*10^-3 0.1904 0.986 9.5