Faculty Presentation - Peter.ppt
- 1. PHASE CHANGES IN HEAT TRANSFER Presented by Mr. J. PETER FERNANDES Assistant Professor Department Mechanical Engineering St. JosephŌĆÖs College of Engineering and Technology Phase Changes in Heat Transfer 1 Mr. J.PETER FERNANDES
- 2. 2 Mr. J.PETER FERNANDES Phase Changes in Heat Transfer ŌĆóBoiling: Boiling is a liquid-to-vapor phase changing process. Boiling occurs at the solidŌĆōliquid interface
- 3. Application of Boiling 3 Mr. J.PETER FERNANDES Phase Changes in Heat Transfer ŌĆó Core and steam generators in nuclear reactors. ŌĆó Petroleum transportation. ŌĆó Electronic cooling and various types of chemical reactors.
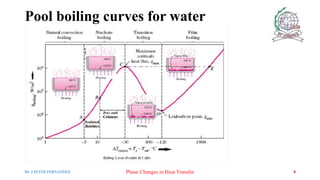
- 4. Pool boiling curves for water 4 Mr. J.PETER FERNANDES Phase Changes in Heat Transfer
- 5. Natural Convection ( Point A on the Boiling Curve) ŌĆó Bubbles do not form on the heating surface until the liquid is heated a few degrees above the saturation temperature (about 2 to 6┬░C for water) the liquid is slightly superheated in this case (meta stable state). ŌĆó The fluid motion in this mode of boiling is governed by natural convection currents. ŌĆó Heat transfer from the heating surface to the fluid is by natural convection. Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 5
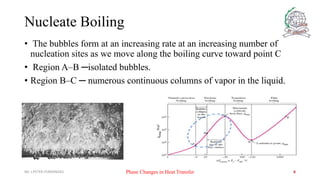
- 6. Nucleate Boiling ŌĆó The bubbles form at an increasing rate at an increasing number of nucleation sites as we move along the boiling curve toward point C ŌĆó Region AŌĆōB ŌöĆisolated bubbles. ŌĆó Region BŌĆōC ŌöĆ numerous continuous columns of vapor in the liquid. Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 6
- 7. Nucleate Boiling ŌĆó In region AŌĆōB the stirring and agitation caused by the entrainment of the liquid to the heater surface is primarily responsible for the increased heat transfer coefficient. ŌĆó In region AŌĆōB the large heat fluxes obtainable in this region are caused by the combined effect of liquid entrainment and evaporation. After point B the heat flux increases at a lower rate with increasing DT excess, and reaches a maximum at point C. ŌĆó The heat flux at this point is called the critical (or maximum) heat flux, and is of prime engineering importance. Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 7
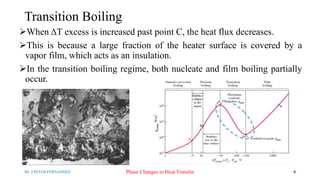
- 8. Transition Boiling ’āśWhen ╬öT excess is increased past point C, the heat flux decreases. ’āśThis is because a large fraction of the heater surface is covered by a vapor film, which acts as an insulation. ’āśIn the transition boiling regime, both nucleate and film boiling partially occur. Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 8
- 9. Film Boiling ŌĆó Beyond Point D the heater surface is completely covered by a continuous stable vapor film. ŌĆó Point D, where the heat flux reaches a minimum is called the Leiden frost point. The presence of a vapor film between the heater surface and the liquid is responsible for the low heat transfer rates in the film boiling region. The heat transfer rate increases with increasing excess temperature due to radiation to the liquid. Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 9
- 10. Pool boiling curves for water-Animation Mr. J.PETER FERNANDES Phase Changes in Heat Transfer 10
- 11. Thank you Phase Changes in Heat Transfer 11 Mr. J.PETER FERNANDES