FBD ppt
- 2. CONTENTS ’āś Introduction. ’āś Construction of Fluidized Bed Dryer (FBD). ’āś Working of FBD. ’āś Validation of FBD. ’āś Validation protocol . ’āś Design Qualification (DQ). ’āś Installation Qualification (IQ). ’āś Operational Qualification (OQ). ’āś Performance Qualification (PQ). ’āś Conclusion. ’āś References. 2
- 3. ’āś Fluid bed drying is most widely used technique for drying Pharmaceutical powders , granules and slurries ’āś Fluid bed processing involves drying, mixing, granulation, and coating of particulate materials. ’āś In fluid bed drying, heat is supplied by the fluidization gas, but the gas flow need not be the only source. Heat may be effectively introduced by heating surfaces (panels or tubes) immersed in the fluidized layer. 3
- 4. contdŌĆ” ’āś The direct contact between particles and air/gas is possible in fluid bed system. ’āś Fluid bed drying is suited for powders, granules, agglomerates, and pellets with an average particle size normally between 50 to 5,000 microns. Very fine, light powders or highly elongated particles may require vibration for successful fluid bed drying. ’āś Materials with moisture content up to 80% such as some polymers, dye stuffs and molecular sieve catalysts can also be accommodated. 4
- 5. ’ā╝ Fluidized bed dryer requires less time to complete drying. i.e., 20 to 40 min. ’ā╝ Hot spots are not observed in the dryer, because of its excellent mixing and drying capacities. ’ā╝ The thermal efficiency is 2 to 6 times greater than tray dryer. ’ā╝ It facilitates the drying of thermolabile substances , since the contact time for drying is short. 5
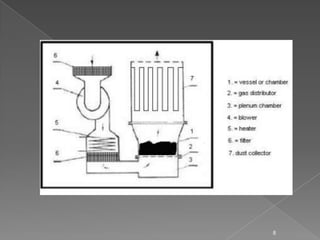
- 6. ’āś The dryer is made up of stainless steel or plastic. ’āś It consist of a hollow vertical chamber where dry, heated air enters through the bottom of the chamber and exhaust air exits through the top of the chamber. ’āś A detachable bowl is placed at the bottom of the dryer, which is used for charging and discharging of material. 6
- 7. ’āś The bowl has a perforated bottom with a wire mesh support for placing materials to be dried and to diffuse the upward flowing air which helps to create a stable and uniform fluidized bed. ’āś A fan is mounted in the upper part for circulating hot air. ’āś Fresh air inlet, pre-filter and heat exchanger are connected serially to heat the air to the required temperature. ’āś Bag filters are placed above the drying bowl for the recovery of fines. 7
- 8. 8
- 9. ’āś The main principle involved in fluidized bed dryer is ŌĆ£FluidizationŌĆØ. ’āś In this state the granules are completely suspended in a stream of hot air which is being supplied from the bottom of the container. ’āś Fluidization produces full agitation of solid particles and since each particle gets surrounded by hot air, heat transfer is extremely high and uniform. 9
- 10. ’āś The product is dried fast without appreciable loss of heat. Filter bags prevent particles escaping from the dryer. ’āś The material is left for some time in the dryer for reaching ambient temperature. ’āś The end product is free flowing. ’āś Warning:- The FBD is not an explosion proof instrument and therefore samples having volatile compounds that are flammable or able to reach their flash point should not be used with this dryer. 10
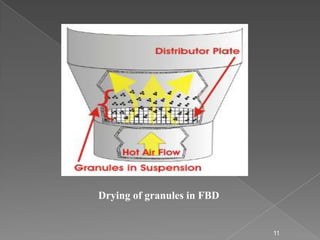
- 11. Drying of granules in FBD 11
- 12. Fixed parameters: ’é¦ Porosity of filter bags, ’é¦ Bowl sieve. Variable ( to be monitored): ’é¦ Inlet/exhaust air temperature, ’é¦ Product temperature, ’é¦ Drying time, ’é¦ Air volume, ’é¦ Humidity of incoming air and exhaust air. 12
- 13. VALIDATION ŌĆ£ Establishing documented evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.ŌĆØ Validation applies to processes or analytical methods. 12
- 14. ’āś Validation provides an approach to prove quality, functionality and performance of a pharmaceutical/biotechnological manufacturing process. ’āś This approach can be applied to individual pieces of equipment as well as the manufacturing process as a whole. 14
- 15. QUALIFICATION ŌĆ£Qualification is a process of assurance that the specific system, premises or equipment are able to achieve the predetermined acceptance criteria to confirm the attributes what it purports to do.ŌĆÖŌĆÖ Qualification applies to equipment. 15
- 16. Equipment Qualification: Studies which establish with confidence that the process equipment and ancillary systems are capable of consistently operating within established limits and tolerances. The studies must include equipment specifications, installation qualification (IQ), operational qualification (OQ) and performance qualification all major equipment to be used in the manufacture of commercial scale batches. Equipment qualification should simulate actual production conditions, including "worst case"/ stressed conditions. Worst Case Condition: The highest and /or lowest value of a given parameter actually evaluated in the validation exercise. 16
- 17. ŌĆó A Validation Protocol is an approved document which outlines the program to be employed, the tests that will be made and the acceptance criteria for those tests. Validation Phases: ’āś DQ- Design Qualification ’āś IQ- Installation Qualification ’āś OQ-Operational Qualification ’āś PQ- Performance Qualification
- 18. DESIGN QUALIFICATION ’āś Documented verification that the proposed design of equipment/systems is suitable for the intended purpose. ’āś Requirements are best created before ŌĆ£shoppingŌĆØ is undertaken. ’āś Requirements are created by the needs of the system/process of which the equipment/system will be a part. 18
- 19. INSTALLATION QUALIFICATION ’āś ŌĆ£Assurance that the intended equipment is received as designed and specifiedŌĆØ. ’āś Verifying proper installation of utilities; water, steam, electrical, compressed air, ventilation, etc. ’āś Instruments for measuring temperature, humidity, time, air volume , pressure as well as recording devices for these variables should be calibrated. 19
- 20. OPERATIONAL QUALIFICATION ’āś The documented evidence that the system or equipment performs as intended throughout all anticipated operating ranges. ’āś ŌĆ£Confirmation that the equipment functions as specified and operates correctlyŌĆØ. ’āś Verifies correct operation of critical components and operating ranges as defined by the specification and required performance. ’āś Operational Tests: Empty Chamber Mapping 20
- 21. PERFORMANCE QUALIFICATION ’āś The documented evidence that the system, equipment or process is capable of consistently producing a safe product of high quality. ’āś Tests to demonstrate that the equipment/system performs in an actual as-used scenario. ŌĆ║ Heat Distribution Studies ŌĆ║ Heat Penetration Studies 21
- 22. ’āś Design qualification is the documentation of the planning phase, including the decision making for the equipment. ’āś The goal is to perform something similar to a risk analysis and to check the design documents of a technical system to ensure that it fulfils the user requirements. ’āś Design qualification takes place before the equipment is constructed. The risk analysis is often part of the design qualification. The earlier risks can be recorded and evaluated, the sooner their minimisation can be taken into consideration in the equipment construction phase. 22
- 23. ’āś In fluidized bed dryer the design of the instrument should be: ’ā╝ Should occupy the small place. ’ā╝ Based on our requirement we can go for horizontal or vertical. ’ā╝ All technical considerations should be kept in mind while doing the design. ’ā╝ Every fluid bed dryer must have explosion relief device of required size 23
- 24. Installation Qualification for fluidized bed dryer include the following steps: ’ā╝ Verifying the approved purchase order. ’ā╝ Verify model number, serial number. ’ā╝ Ensure that all relevant documentation is received: User manual, Maintenance manual, List of change parts, Electrical drawings. 24
- 25. ’ā╝ Check the manufacturer and supplier. ’ā╝ Check for any physical damage. ’ā╝ Confirm location and installation requirements per recommendation of manufacturer. ’ā╝ Verify that the utilities required are available. ’ā╝ Dust free area and moisture free air should be provided. ’ā╝ Installation shall be conducted per instructions provided in the manual. 25
- 26. ’āś Verify alarm control. ’āś Operate the equipment at low, medium, and high speed per operations manual to verify the operating control. ’āś Verify that all switches and push buttons are functioning properly. ’āś Establish procedures for operation, maintenance, and calibration. ’āś Establish training program for relevant staff. ’āś All the electrical fittings in the room must conform to ISI specifications of Flameproof Electrical fittings. ’āś Do the tests for uniform distribution of air. 26
- 27. Run three batches of each product and analyze for: ’āś Active ingredients homogeneity. ’āś Moisture content. ’āś Particle size distribution. ’āś Percentage fines. Based on this data we can fix drying end points for each process. 27
- 28. Specially for FBD: i. Air temperature distribution: Using thermocouples. ii. Inlet air installation: a) Delay time for achieving constant air conditions: Using thermocouple and hygrometer. b) Microbiological quality of the inlet air: Using centrifugal air sampler. 28
- 29. It is check up of what we want actually for that particular process from the equipment. i. Inlet air speed. ii. Quality of air. iii. Uniform distribution of air. iv. Mixing of air with temperature. Run the trial batch during operation and there should not be any change in the: 1. Size, 2. Shape , 3.Surface characteristics of the material which we kept for drying. 29
- 30. ’é¦ Validation provides an approach to prove quality, functionality and performance of a pharmaceutical/biotechnological manufacturing process. ’é¦ Each time before use of the equipment it should be calibrated and maintained and proper precautions should be taken to increase the life span of the equipment. 30
- 31. ’é× C.V.S. Subrahmanyam et al, page no. 396-398,Pharmaceutical Engineering. ’é× Development Pharmaceutics and Process Validation, CPMP, 1988. ’é× Guidelines on the Validation of Manufacturing Process, WHO, 1996. ’é× Principles of Qualification and Validation in Pharmaceutical Manufacture, PIC, 1996. ’é× www.google.co.in 31
- 32. ’é× www.pharmainfo.net ’é× www.ikev.org/haber/bozzonejune1.pdf ’é× www.pharmweb.net ’é× en.wikipedia.org/wiki/Validation ’é× www.ikev.org/haber/inspection/.../ Turkey Qualification_Validation.pdf ’é× http://www.qclabequipment.com/Sherwood_Digital___Analo gue.pdf 32
- 33. 33
Editor's Notes
- #12: Drying of granules in FBD