Masters Thesis Defense Presentation
- 1. ELECTRODEPOSITED GIANT MAGNETORESISTANCE IN FeCoNiCu/Cu AND CrFeCoNiCu/Cu MULTILAYERED NANOWIRESPRAJON RAJ SHAKYA, B.E.M.S. in ELECTRICAL ENGINEERINGADVISOR: Dr. DESPINA DAVIS16th December, 2010
- 2. OverviewMotivation of the ResearchDiscovery and Historical perspectiveIntroductionRelated ResearchExperimental DetailsResults and DiscussionsConclusion and Future WorkReferencesAcknowledgements
- 3. 1. Motivation Higher areal density of storage (write) and sensing (read) devices are always in demand.
- 4. The increase of storage density of 0.132Gb/inch2 in 1991 to 500Gb/inch2 as of today.[1]
- 5. Small size with high storage capacity demands the fabrication in nanostructures.
- 6. Electrodeposition is an economical method and can fabricate the nanowires with high aspect ratio.
- 7. Sensing devices require high sensitivity (low coercivity), high speed and low magnetic saturation field.Project GoalInvestigate fabrication of nanowires for better sensitivity, low coercivity and magnetic saturation by the method of electrodeposition.Device Property Transition elements (3d group) having free electron in outer shell are used for the fabrication of these devices.
- 9. Electrons spin are aligned parallel to each other.
- 10. Magnetization is intact even after removal of external magnetic field.
- 12. Electrons are arranged in antiparallel to each other.
- 13. Magnetoresistance (MR) ratio is defined as the ratio of the change in resistivity (due to change in configuration of electrons from antiparallel to parallel) to the resistivity in parallel configuration of electrons.
- 14. MR is due to spin of electrons and was first observed in ferromagnetic materials as AMR (Anisotropic Magnetoresistance).
- 15. AMR shows increase in resistance along and decrease in resistance across the lines of magnetization. [2]2. Discovery and Historical Perspective This effect of MR was also observed in multilayers with alternating layer of ferromagnetic (FM) and Non Magnetic (NM) layer as GMR (Giant Magnetoresistance).
- 16. Fert et al. and Grunberg et al. were first to observe GMR in Fe/Cr multilayers with the method of MBE (Molecular Beam Epitaxy). [1, 3, 4 ]Figure 1: Giant Magnetoresistance in (a) Fe/Cr multilayers by Fert et al. (left) [1, 3] and (b) Fe/Cr/Fe tri layers by Grunberg et al. (right) [1, 4]
- 17. 3. Introduction GMR is the effect that refers to the change in resistance of a material when an applied magnetic field changes electron spin alignment of the ferromagnetic layer from antiparallel to parallel.Figure 2: Schematic diagram of GMR Effect (left) and schematic representation of GMR using simple resistor model (right) [1]
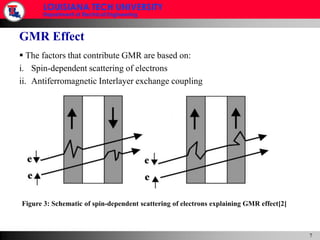
- 18. GMR Effect The factors that contribute GMR are based on:Spin-dependent scattering of electrons Antiferromagnetic Interlayer exchange couplingFigure 3: Schematic of spin-dependent scattering of electrons explaining GMR effect[2]
- 19. Measurement Geometries Current In Plane (CIP) and Current Perpendicular to the Plane (CPP) GMR.
- 20. Characteristic length = mean free path of electrons (╬╗) (CIP GMR)
- 21. Characteristic length = spin diffusion length (L)(CPP GMR)
- 22. L>> ╬╗Figure 4: Schematic Diagram of CIP and CPP GMR (left) with measurement techniques(right) [1]
- 23. 4. Related Research Bivalent and trivalent system
- 24. Piraux et al. was first to study GMR in electrodeposited multilayered nanowires. They observed around 15% GMR at room temperature in Co/Cu layers. [5]
- 25. Blondel et al. observed GMR of 14% for Co/Cu and 10% for FeNi/Cu multilayered nanowires. [6]
- 26. Liu et al. investigated Co/Cu multilayerand observed GMR of 11% at room temperature and 22% at 5K . [7]
- 27. CoFeCu/Cu multilayered nanowires were studied by Seyama et al. and observed CPPGMR was twice that of CIP GMR on thin film for the same elements.[8]
- 28. Kakuno et al. also studied CoFeCu/Cu but with compositionally modulated alloys and observed 8% GMR at room temperature with polycrystalline deposit of face centered cubic and hexagonal close packed structures. [9]CoNiCu/Cu multilayers
- 29. Blondel et al. studied CoNi/Cu multilayered nanowires by pulsed potential technique and observed 20% GMR at room temperature with same ferromagnetic and nonmagnetic layer thickness. [10]
- 30. Schwarzacher et al. and Heydon et al. investigated CoNiCu/Cu in polycarbonate membranes and observed 22% GMR at room temperature. The reduction in the dissolution of Co was observed in the deposition of Cu with the addition of Ni.[11]
- 31. Evans et al. reported 55% GMR at room temperature and 115% GMR at low temperature on AAO with CoNiCu/Cu multilayers. They also reported that better GMR was observed with AAO template than with PC membranes. [12]
- 33. Huang and Podlaha investigated quaternary system of FeCoNiCu and observed 4% GMR at 300K and 18% at 4K with a Cu layer thickness of 1.8nm. Anodic dissolution during multilayer deposition at low potential pulse was observed and galvanostatic triple pulses with relaxation period were introduced to reduce it. [13]
- 34. J. Gong et al. studied sensitivity of FeCoNiCu/Cu multilayers and observed decrease in coercivity with increase Fe concentration. They reported 9% GMR saturated at less than 0.5KOe and sensitivity of over 0.11% Oe. [14]Electrodeposition of Cr
- 35. Dolati et al. studied FeCrNiMo alloys using chloride electrolyte and reported increase in Cr content increased the current density. They also observed fine-grain, smooth and compact deposits of FeCrNiMo. [15]
- 36. Xin-Quai et al. investigated pulse electrodeposition of Cr from trivalent bath and reported that thicker coatings and finer grains were observed with lower temperature and current density. [16 ]
- 37. Lallemandet al. studied electrodeposition of soft CoFeCr films and reported that the addition of Cr increases the resistivity of the alloy. [17]
- 38. Ericksson et al. investigated the effect of addition of chromium in FeNi alloy. They observed the improvement in crystal anisotropy with improved texture and small grain size that resulted in the decrease of saturation magnetization. [18]
- 39. Choi et al. studied the effect of Cr addition on structure as well as the corrosion resistance in FeTiN nanocrystalline thin films and observed the reduction in coercivity. They also showed that Cr tends to form passivation layer and helps to minimize corrosion [19] .5. Experimental Details - Setup Electrodeposition was the technique used.
- 40. Cathode: Gold sputtered AAO membrane
- 41. Anode: Platinum Mesh
- 42. Reference: Saturated Calomel Electrode (SCE)M1-> M1n+ + ne- (oxidation)M2n+ + ne- -> M2 (reduction)Figure 6: Schematic view of electrodeposition setup (left) and SEM picture of AAO membrane (right)
- 43. Electrochemical System Deposition of metals takes place at reduction phase of redox reaction.
- 44. Two distinct mechanism:
- 45. Kinetic Control : Cs = Cb Mass Transport Control: Cs < Cb In Cr-Fe-Co-Ni-Cu system; Cu>Ni>Co>Fe>Cr deposition
- 46. Anomalous codeposition in Fe group elements so Fe>Co>Ni.Figure 5: Schematic diagram showing electrodeposition mechanism (left) and deposition of Cu, Fe group elements and Cr (right)
- 47. Electrodeposition Techniques Constant potential: alloy or elemental nanowires
- 48. Pulsed potential: multilayered nanowiresCu topCu layerAlloy layerCu bottomFigure 7: Schematic view of electrodeposition technique
- 49. Measurement TechniquesFigure 8: Lakeshore 7700 Hall Effect Measurement System for GMR measurement (left) and Alternating Gradient Magnetometer (AGM) Micromag 2900 for magnetic measurements (right)
- 50. 6. Results and DiscussionsElectrolyte CharacterizationTable 1: Molar Concentration of baths used for electrodeposition0
- 51. Polarization study Shows the peaks as the identification of deposition region of elements.
- 52. Cu deposition range is identified as -0.25V to -0.4V and alloy deposition range as -1.4V to -2.6V.
- 53. Current density is increased with the addition of Cr which is due to increase of number of ions and hence conductivity.Figure 9:Polarization resistance curve for bath A and bath B at sweep rate of 2mvps
- 54. Compositional Analysis Bath A was used to determine optimal Cu potential.
- 55. Range of Cu potentials from -0.25V to -0.4V were investigated.
- 56. Optimal Cu deposition potential was identified at -0.3V.Table 2: Compositional Analysis for bath A at lower Cu potentialsFigure 10: Compositional Analysis for bath A for identification of optimal Cu potential
- 57. Compositional Analysis of bath A for alloy potential For bath A, analysis was done for potentials from -1.4V to -2.6V.
- 58. 20nm AAO membrane was used for constant potential electrodeposition. Table 3: Compositional Analysis of bath A at different alloy potentialsFigure 11: Compositional Analysis for bath A at different alloy potentials
- 59. Compositional Analysis of bath B for alloy potential Similar range of alloy potentials from -1.4V to -2.6V were analyzed.
- 60. Co composition was found highest at all potentials.
- 61. Ni composition was found increasing and Cu decreasing with the alloy potential.Table 4: Compositional Analysis of bath B at different alloy potentialsFigure 12: Compositional Analysis for bath B at different alloy potentials
- 62. GMR Results in FeCoNiCu/Cu nanowiresEffect of change of alloy potential on bath AMaximum GMR of 10.64% was obtained at alloy potential of -2.2V. Saturation field was high and linearly proportional to GMR curve.Figure 13: Optimum GMR on varying alloy potential (left) and relation with Saturation field (T) with the change of alloy potential (right)
- 63. Effect of change of alloy potential time (alloy thickness) on bath A Optimum alloy potential of -2.2V was chosen.
- 64. Beside alloy potential time all other parameters were kept constant.
- 65. Maximum GMR obtained was 10.64% for alloy potential time of 1sec. (alloy thickness) where saturation field was the lowest.Figure 14: Optimum GMR on varying alloy pulsing time (left) and relation with Saturation field (T) with the change of alloy potential time (right)
- 66. Effect of change of number of layers on bath A Optimum alloy potential of -2.2V and optimal alloy pulsing time of 1 sec was selected and the number of layers were varied from 500 to 2500 bilayers.
- 67. GMR increased with the increase of number of bilayers and maximum of 14.56% at 2500 layers . Low saturation field was observed at this GMR. .Figure 15: Optimum GMR with varying number of layers (left) and relation with Saturation field (T) with the change of number of layers (right)
- 68. GMR Results in CrFeCoNiCu/Cu nanowiresEffect of change of alloy potential on bath B Optimal Cu potential of -0.3V and other parameters were kept constant.
- 69. Maximum GMR observed was 5.62% at an alloy potential of -2.2V where the lowest saturation field was observed.Figure 16: Optimum GMR on varying alloy potential (left) and relation with Saturation field (T) with the change of alloy potential (right)
- 70. Effect of change of alloy potential time (alloy thickness) on bath B1000 bilayers were deposited at optimum alloy potential of -2.2V.
- 71. Maximum GMR of 5.62% was observed at an alloy potential time of 1sec and lowest saturation field was observed at that point.Figure 17: Optimum GMR on varying alloy pulsing time (left) and relation with Saturation field (T) with the change of alloy potential time (right)
- 72. Effect of change of number of layers on bath BGMR increases with the increase in number of bilayers with maximum GMR of 5.82% at 2500 layers which even had the lowest saturation field.Figure 18: Optimum GMR with varying number of layers (left) and relation with Saturation field (T) with the change of number of layers (right)
- 73. SEM Images of Multilayered Nanowires Cu potential of -0.3V and alloy potential of -2.2V were used for pulse potential electrodeposition.
- 74. Cu pulsing time was kept for 100secs and alloy pulsing time for 20secs.
- 75. 20nm pore size AAO and 200nm pore size PC were used as substrate.
- 76. Dichloromethane was used for dissolving membrane and liberating nanowires in case of PC template.
- 77. AAO template was dissolved in saturated 1M NaOH, centrifuged and washed several times to liberate the nanowires for measurement. Alternating dark and gray layers were observed.
- 78. Dark layer were identified as Cu with thickness ranging from 28-40nm and gray alloy layer had thickness range from 72-90nm in AAO and 90-105nm in PC.Figure 19: SEM images of CrFeCoNiCu/Cu nanowires on 20nm AAO (left) and 200nm PC (right)
- 79. Effect of Chromium on GMR Both bath A and B showed similar trends.
- 80. Highest GMR was observed at -2.2V, 1sec and 2500 layers.
- 81. The presence of Cr in the alloy layer decreased GMR because Cr being non-magnetic hinders the anti-ferromagnetic interlayer exchange coupling that is responsible for GMR.Figure 20: Variation of GMR for bath A and B with (a) change of alloy potential (left) (b) change of Alloy potential time (right-top)(c) number of layers (right-bottom)
- 82. Effect of Chromium on Coercivity The point of highest GMR showed the lowest coercivity.
- 83. With the addition of Cr, coercivity is reduced because Cr is non-magnetic and smoother deposits with finer grain size deposition of Cr reduces coercivity.Figure 21: Variation of Coercivity for bath A and B with (a) change of alloy potential (left) (b) change of alloy potential time (right-top)(c) number of layers (right-bottom)
- 84. 7. Conclusion Highest GMR was observed at optimum alloy potential of -2.2V and optimum alloy pulsing time of 1 sec.
- 85. Increase in number of bilayers increased GMR percentage with highest GMR obtained as 14.56% for FeCoNiCu/Cu and 5.82% for CrFeCoNiCu/Cu nanowires at 2500 layers.
- 86. Highest GMR curves tended to saturate faster which is desirable for read sensors.
- 87. Addition of Cr on the alloy region tended to decrease the GMR because Cr being non magnetic and its presence in ferromagnetic region deteriorated interlayer exchange coupling phenomena.
- 88. Coercivity of the nanowires was lowered with the addition of Cr because of the regular, granular deposition of Cr that shows proper magnetic interaction between nanowires. Least coercivity values was observed with highest GMR values.Future Work Cr is anti-corrosive in nature and its effect on corrosion of nanowires will be studied .
- 89. Variation of Cr concentration and effect of temperature will be explored in the field of CPP GMR magnetic sensors.References[1] S. M. Thompson, ŌĆ£The Discovery, Development and Future of GMR: The Nobel Prize 2007,ŌĆØ Journal of Physics D: Applied Physics, vol. 41, no. 9, pp. 1-20, 28th March 2008.[2] A. Schuhl, and D. Lacour, ŌĆ£Spin Dependent Transport: GMR & TMR,ŌĆØ ComptesRendus Physique, vol. 6, no. 9, pp. 945-955, November 2005.[3] M. N. Baibich, J. M. Broto, A. Fert, F. Nguyen Van Dau, F. Petroff, P. Eitenne, G. Creuzet, A. Friederich and J. Chazelas, ŌĆ£Giant Magnetoresistance of (001) Fe/ (001) Cr Magnetic Superlattices,ŌĆØ Physical Review Letters, vol. 61, no. 21, pp. 2472-2475, 21st November 1988.[4] G. Binasch, P. Grunberg, F. Saurenbach and W. Zinn ŌĆ£Enhanced Magnetoresitance in Layered Magnetic Structures with Antiferromagnetic Interlayer Exchange,ŌĆØ Physical Review B, vol. 39, no.7, pp. 4828- 4830, 1st March 1989.[5] L. Piraux, J. M. George, J. F. Despres, C. Leroy, E. Ferain, R. Legras, K. Ounadjela and A. Fert, ŌĆ£Giant Magnetoresistance in Magnetic Multilayered Nanowires,ŌĆØ Applied Physics Letters, vol. 65, no. 19, pp. 2484-2486, 7th November 1994.[6] A. Blondel, J. P. Meier, B. Doudin and J. ŌĆōPh. Ansermet, ŌĆ£Giant Magnetoresistance of Nanowires of Multilayers,ŌĆØ Applied Physics Letters, vol. 65, no. 23, pp. 3019-3021, 5th December 1994.[7] K. Liu, K. Nagodawithana, P. C. Searson and C. L. Chien, ŌĆ£Perpendicular Giant Magnetoresistance of Multilayered Co/Cu Nanowires,ŌĆØ Physical Review B, vol. 51, no. 11, pp. 7381-7384, 15th March 1995.[8] Y. Seyama, A. Tanaka and M. Oshiki, ŌĆ£Giant Magnetoresistance Properties of CoFe/Cu Multilayer in the CPP (Current Perpendicular to the Plane) Geometry,ŌĆØ IEEE Transactions on Magnetics, vol. 35, no. 5, pp. 2838-2840, September 1999.[9] E. M. Kakuno, R. C. da Silva, N. Mattoso, W. H. Schreiner, D. H. Mosca and S. R. Teixeira, ŌĆ£Giant Magnetoresistance in Electrodeposited Co87Fe13/Cu compositionally Modulated Alloys,ŌĆØ Journal of Physics D: Applied Physics, vol. 32, no. 11, pp. 1209-1213, 7th June 1999.
- 90. [10] A. Blondel, J. Meier, B. Doudin, J. ŌĆōPh. Ansermet, K. Attenborough, P. Evans, R. Hart, G. Nabiyouni and W. Schwarzacher, ŌĆ£Wire-shaped Magnetic Multilayers for ŌĆśCurrent Perpendicular to PlaneŌĆÖ Magnetoresistance Measurements,ŌĆØ Journal of Magnetism and Magnetic Materials, vol. 148, no.1-2, pp. 317-318, 1st July 1995.[11] G. P. Heydon, S. R. Hoon, A. N. Farley, S. L. Tomlinson, M. S. Valera, K. Attenborough and W. Schwarzacher, ŌĆ£Magnetic Properties of Electrodeposited Nanowires,ŌĆØ Journal of Physics D: Applied Physics, vol. 30, no. 7, pp. 1083-1093, 7th April 1997.[12] P. R. Evans, G. Yi and W. Schwarzacher, ŌĆ£Current Perpendicular to Plane Giant Magnetoresistance of Multilayered Nanowires Electrodeposited in Anodic Aluminum Oxide Membranes,ŌĆØ Applied Physics Letters, vol. 76, no. 4, pp. 481-483, 24th January 2000.[13] Q. Huang, D. P. Young and E. J. Podlaha, ŌĆ£Magnetoresistance of Electrodeposited Iron-Cobalt-Nickel-Copper Multilayers,ŌĆØ Journal of Applied Physics, vol. 94, no. 3, pp. 1-4, 1st August 2003.[14] J. Gong, W. H. Butler, G. Zangari, ŌĆ£Optimization of Magnetoresistive Sensitivity in Electrodeposited FeCoNi/Cu Multilayers,ŌĆØ IEEE Transactions on Magnetics, vol. 41, no. 10, pp. 3634-3636, October 2005.[15] A. G. Dolati, M. Ghorbani and A. Afshar, ŌĆ£The Electrodeposition of Quaternary Fe-Cr-Ni-Mo Alloys from the Chloride-Complexing Agents Electrolyte. Part I. Processing,ŌĆØ Surface and Coatings Technology, vol. 166, no. 2-3, pp. 105-110, 24th March 2003.┬Ā[16] H. Xin-Kuai, Q. Guan-Zhou, C. Bai-zhen, Z. Ning-bo, W. Lu-ye and X. Li-jian, ŌĆ£Process of Pulse Electrodeposition Nanocrystalline Chromium From Trivalent Chromium Bath,ŌĆØ Transactions of Nonferrous Metals Society of China, vol. 17, pp. s685-s691, 2007.[17] F. Lallemand, D. Comte, L. Ricq, P. Renaux, J. Pagetti, C. Dieppedale and P. Gaud, Effects of organic additives on electroplated soft magnetic CoFeCr films,ŌĆØ Applied Surface Science, vol. 225, no. 1-4, pp. 59-71, 2004.[18] H. Eriksson and A. Salwen, ŌĆ£50 Permalloy alloyed with Cr for increasing corrosion resistanceŌĆØ, IEEE Transactions on Magnetics, vol. 13, no.5, 1977.[19] H. W. Choi, K. H. Kim, J. Kim, S. H. Han and H. J. Kim, ŌĆ£The Effect of Cr Addition on Structure and Corrosion Resistance in FeTiN Nanocrystalline Soft Magnetic Thin Films,ŌĆØ IEEE Transactions on Magnetics, vol. 37, no. 4, July 2001.
- 94. Dr. DentchoGenov
- 95. Group Members
- 96. Family and FriendsTHANK │█░┐▒½’üŖ



![The increase of storage density of 0.132Gb/inch2 in 1991 to 500Gb/inch2 as of today.[1]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-4-320.jpg)









![AMR shows increase in resistance along and decrease in resistance across the lines of magnetization. [2]2. Discovery and Historical Perspective This effect of MR was also observed in multilayers with alternating layer of ferromagnetic (FM) and Non Magnetic (NM) layer as GMR (Giant Magnetoresistance).](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-15-320.jpg)
![Fert et al. and Grunberg et al. were first to observe GMR in Fe/Cr multilayers with the method of MBE (Molecular Beam Epitaxy). [1, 3, 4 ]Figure 1: Giant Magnetoresistance in (a) Fe/Cr multilayers by Fert et al. (left) [1, 3] and (b) Fe/Cr/Fe tri layers by Grunberg et al. (right) [1, 4]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-16-320.jpg)
![3. Introduction GMR is the effect that refers to the change in resistance of a material when an applied magnetic field changes electron spin alignment of the ferromagnetic layer from antiparallel to parallel.Figure 2: Schematic diagram of GMR Effect (left) and schematic representation of GMR using simple resistor model (right) [1]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-17-320.jpg)
![GMR Effect The factors that contribute GMR are based on:Spin-dependent scattering of electrons Antiferromagnetic Interlayer exchange couplingFigure 3: Schematic of spin-dependent scattering of electrons explaining GMR effect[2]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-18-320.jpg)



![L>> ╬╗Figure 4: Schematic Diagram of CIP and CPP GMR (left) with measurement techniques(right) [1]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-22-320.jpg)

![Piraux et al. was first to study GMR in electrodeposited multilayered nanowires. They observed around 15% GMR at room temperature in Co/Cu layers. [5]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-24-320.jpg)
![Blondel et al. observed GMR of 14% for Co/Cu and 10% for FeNi/Cu multilayered nanowires. [6]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-25-320.jpg)
![Liu et al. investigated Co/Cu multilayerand observed GMR of 11% at room temperature and 22% at 5K . [7]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-26-320.jpg)
![CoFeCu/Cu multilayered nanowires were studied by Seyama et al. and observed CPPGMR was twice that of CIP GMR on thin film for the same elements.[8]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-27-320.jpg)
![Kakuno et al. also studied CoFeCu/Cu but with compositionally modulated alloys and observed 8% GMR at room temperature with polycrystalline deposit of face centered cubic and hexagonal close packed structures. [9]CoNiCu/Cu multilayers](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-28-320.jpg)
![Blondel et al. studied CoNi/Cu multilayered nanowires by pulsed potential technique and observed 20% GMR at room temperature with same ferromagnetic and nonmagnetic layer thickness. [10]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-29-320.jpg)
![Schwarzacher et al. and Heydon et al. investigated CoNiCu/Cu in polycarbonate membranes and observed 22% GMR at room temperature. The reduction in the dissolution of Co was observed in the deposition of Cu with the addition of Ni.[11]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-30-320.jpg)
![Evans et al. reported 55% GMR at room temperature and 115% GMR at low temperature on AAO with CoNiCu/Cu multilayers. They also reported that better GMR was observed with AAO template than with PC membranes. [12]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-31-320.jpg)

![Huang and Podlaha investigated quaternary system of FeCoNiCu and observed 4% GMR at 300K and 18% at 4K with a Cu layer thickness of 1.8nm. Anodic dissolution during multilayer deposition at low potential pulse was observed and galvanostatic triple pulses with relaxation period were introduced to reduce it. [13]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-33-320.jpg)
![J. Gong et al. studied sensitivity of FeCoNiCu/Cu multilayers and observed decrease in coercivity with increase Fe concentration. They reported 9% GMR saturated at less than 0.5KOe and sensitivity of over 0.11% Oe. [14]Electrodeposition of Cr](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-34-320.jpg)
![Dolati et al. studied FeCrNiMo alloys using chloride electrolyte and reported increase in Cr content increased the current density. They also observed fine-grain, smooth and compact deposits of FeCrNiMo. [15]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-35-320.jpg)
![Xin-Quai et al. investigated pulse electrodeposition of Cr from trivalent bath and reported that thicker coatings and finer grains were observed with lower temperature and current density. [16 ]](https://image.slidesharecdn.com/finalthesisdefenseppt2-13077481902825-phpapp02-110610182343-phpapp02/85/Masters-Thesis-Defense-Presentation-36-320.jpg)