Gas chromatography (1)
- 1. GAS CHROMATOGRAPHY Prepared By:- Mr. Lokesh Thote Asstt. Professor Department of Pharmaceutical Chemistry Kamla Nehru College of Pharmacy, Butibori, Nagpur (M.S.)
- 2. Introduction * Gas chromatography – It is a process of separating component(s) from the given crude drug or mixture by using stationary phase (solid or liquid) and gaseous mobile phase. * It involves a sample being vaporized and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. * The column itself contains a solid or liquid stationary phase which is adsorbed onto the surface of an inert solid. * Two major types • Gas-solid chromatography (GSC) (Stationary phase: solid) • Gas-liquid chromatography (GLC) (Stationary phase: immobilized liquid)
- 3. Advantages of Gas Chromatography * The technique has strong separation power and even complex mixture can be resolved into constituents * The sensitivity of the method is quite high. it is micro method only few mg of sample is sufficient for analysis. * It gives good precision and accuracy * The analysis is completed in a short time * The cost of instrument is relatively low and its life is generally long * The technique is relatively suitable for routine analysis (do not require highly skilled person for operation)
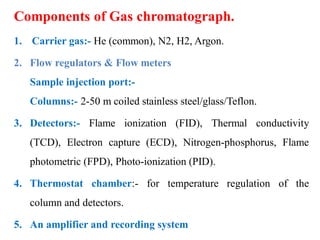
- 4. Components of Gas chromatograph. 1. Carrier gas:- He (common), N2, H2, Argon. 2. Flow regulators & Flow meters Sample injection port:- Columns:- 2-50 m coiled stainless steel/glass/Teflon. 3. Detectors:- Flame ionization (FID), Thermal conductivity (TCD), Electron capture (ECD), Nitrogen-phosphorus, Flame photometric (FPD), Photo-ionization (PID). 4. Thermostat chamber:- for temperature regulation of the column and detectors. 5. An amplifier and recording system
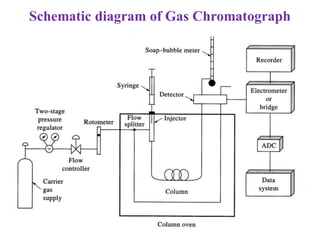
- 5. Schematic diagram of Gas Chromatograph
- 7. 1. Carrier gas The carrier gas must be chemically inert. Commonly used gases include hydrogen, nitrogen, helium, argon, and carbon dioxide. Hydrogen has more advantages as compared to others but it highly flammable. Helium is generally used because of its excellent thermal conductivity, inertness, low density and allow high flow rate. The choice of carrier gas is often dependant upon the type of detector which is used. For selecting carrier following thing should be consider o It should be inert o Should be suitable for detectors. No fire or explosion hazards o Readily available in high purity. Should be cheap.
- 8. 2. Flow regulators & Flow meters Deliver the gas with uniform pressure/flow rate flow meters:- Rota meter & Soap bubble flow meter ROTA METER  Placed before column inlet  It has a glass tube with a float held on to a spring.  The level of the float is determined by the flow rate of carrier gas
- 9. SOAP BUBBLE METER ÔÉòSimilar to Rota meter but instead of a float, soap bubble formed indicates the flow rate
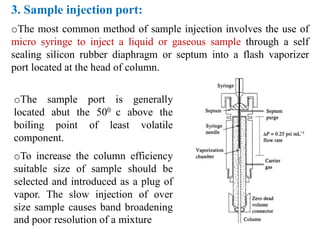
- 10. 3. Sample injection port: oThe most common method of sample injection involves the use of micro syringe to inject a liquid or gaseous sample through a self sealing silicon rubber diaphragm or septum into a flash vaporizer port located at the head of column. oThe sample port is generally located abut the 500 c above the boiling point of least volatile component. oTo increase the column efficiency suitable size of sample should be selected and introduced as a plug of vapor. The slow injection of over size sample causes band broadening and poor resolution of a mixture
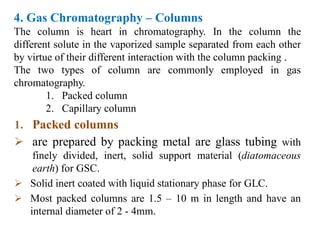
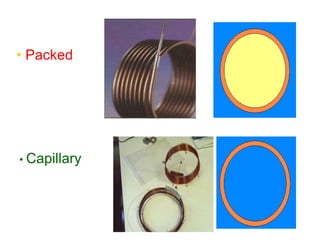
- 11. 4. Gas Chromatography – Columns The column is heart in chromatography. In the column the different solute in the vaporized sample separated from each other by virtue of their different interaction with the column packing . The two types of column are commonly employed in gas chromatography. 1. Packed column 2. Capillary column 1. Packed columns  are prepared by packing metal are glass tubing with finely divided, inert, solid support material (diatomaceous earth) for GSC.  Solid inert coated with liquid stationary phase for GLC.  Most packed columns are 1.5 – 10 m in length and have an internal diameter of 2 - 4mm.
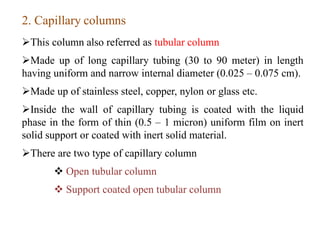
- 12. 2. Capillary columns This column also referred as tubular column Made up of long capillary tubing (30 to 90 meter) in length having uniform and narrow internal diameter (0.025 – 0.075 cm). Made up of stainless steel, copper, nylon or glass etc. Inside the wall of capillary tubing is coated with the liquid phase in the form of thin (0.5 – 1 micron) uniform film on inert solid support or coated with inert solid material. There are two type of capillary column  Open tubular column  Support coated open tubular column
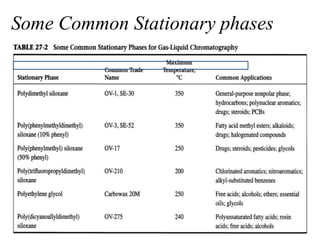
- 14. Some Common Stationary phases
- 15. 5. G C - DETECTORS There are many detectors which can be used in gas chromatography Different detectors will give different types of selectivity. * Detectors can be grouped into concentration dependant detectors and mass flow dependant detectors. * The signal from a concentration dependant detector is related to the concentration of solute in the detector, and does not usually destroy the sample Dilution of with make-up gas will lower the detectors response. * Mass flow dependant detectors usually destroy the sample, and the signal is related to the rate at which solute molecules enter the detector. The response of a mass flow dependant detector is unaffected by make-up gas
- 16. G C – IDEAL DETECTORS Sensitive (10-8-10-15 g solute/s) Operate at high T (0-400 °C) S table and reproducible Linear response Wide dynamic range Fast response Simple (reliable) Nondestructive Uniform response to all analytes
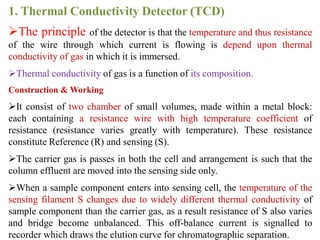
- 17. 1. Thermal Conductivity Detector (TCD) ÔÉòThe principle of the detector is that the temperature and thus resistance of the wire through which current is flowing is depend upon thermal conductivity of gas in which it is immersed. ÔÉòThermal conductivity of gas is a function of its composition. Construction & Working ÔÉòIt consist of two chamber of small volumes, made within a metal block: each containing a resistance wire with high temperature coefficient of resistance (resistance varies greatly with temperature). These resistance constitute Reference (R) and sensing (S). ÔÉòThe carrier gas is passes in both the cell and arrangement is such that the column effluent are moved into the sensing side only. ÔÉòWhen a sample component enters into sensing cell, the temperature of the sensing filament S changes due to widely different thermal conductivity of sample component than the carrier gas, as a result resistance of S also varies and bridge become unbalanced. This off-balance current is signalled to recorder which draws the elution curve for chromatographic separation.
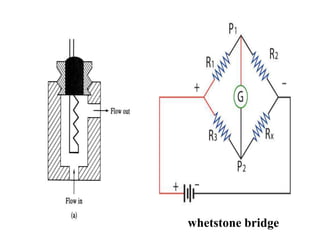
- 18. whetstone bridge
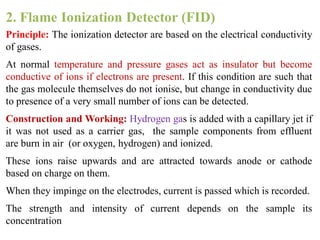
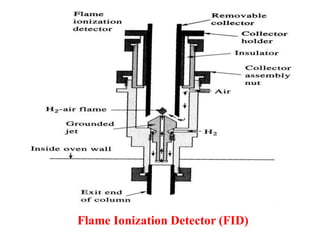
- 19. 2. Flame Ionization Detector (FID) Principle: The ionization detector are based on the electrical conductivity of gases. At normal temperature and pressure gases act as insulator but become conductive of ions if electrons are present. If this condition are such that the gas molecule themselves do not ionise, but change in conductivity due to presence of a very small number of ions can be detected. Construction and Working: Hydrogen gas is added with a capillary jet if it was not used as a carrier gas, the sample components from effluent are burn in air (or oxygen, hydrogen) and ionized. These ions raise upwards and are attracted towards anode or cathode based on charge on them. When they impinge on the electrodes, current is passed which is recorded. The strength and intensity of current depends on the sample its concentration
- 20. Flame Ionization Detector (FID)
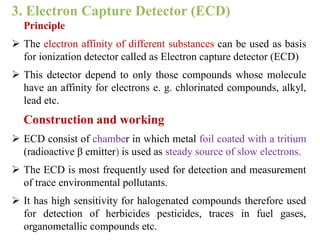
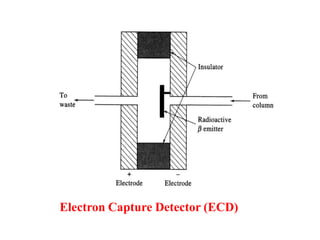
- 21. 3. Electron Capture Detector (ECD) Principle  The electron affinity of different substances can be used as basis for ionization detector called as Electron capture detector (ECD)  This detector depend to only those compounds whose molecule have an affinity for electrons e. g. chlorinated compounds, alkyl, lead etc. Construction and working  ECD consist of chamber in which metal foil coated with a tritium (radioactive β emitter) is used as steady source of slow electrons.  The ECD is most frequently used for detection and measurement of trace environmental pollutants.  It has high sensitivity for halogenated compounds therefore used for detection of herbicides pesticides, traces in fuel gases, organometallic compounds etc.
- 22. Electron Capture Detector (ECD)
- 23. 4. Argon ionization detector; ÔÉòThese detectors are similar to flame ionization detectors with only difference that argon ion gas is used to ionize the sample molecules. ÔÉòThe argon ion are obtained by reacting argon gas with radio active elements. Once argon ionizes they try to get back to stable state by either taking or giving electron from the sample components thus making sample molecules to ions for detection.
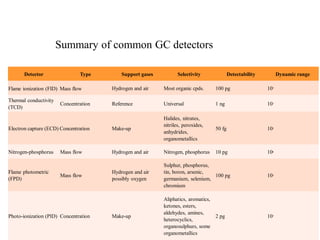
- 24. Summary of common GC detectors Detector Type Flame ionization (FID) Mass flow Support gases Selectivity Detectability Dynamic range Hydrogen and air Most organic cpds. 100 pg 107 Thermal conductivity (TCD) Concentration Reference Universal 1 ng 107 Electron capture (ECD) Concentration Make-up Halides, nitrates, nitriles, peroxides, anhydrides, organometallics 50 fg 105 Nitrogen-phosphorus Mass flow Hydrogen and air Nitrogen, phosphorus 10 pg 106 Flame photometric (FPD) Mass flow Hydrogen and air possibly oxygen Sulphur, phosphorus, tin, boron, arsenic, germanium, selenium, chromium 100 pg 103 Photo-ionization (PID) Concentration Make-up Aliphatics, aromatics, ketones, esters, aldehydes, amines, heterocyclics, organosulphurs, some organometallics 2 pg 107
- 25. 6. Temperature Programming System  As temperature programming facilitates controlled increase of even temperature during analysis. So that peaks of analytes should be sharp and emerge quickly.  Because of temperature programming facility the components of wide boiling point range mixture may be resolved efficiently.  Temperature programming may be carried out by three different modes  Natural or ballastic  Linear  Matrix or multilinear
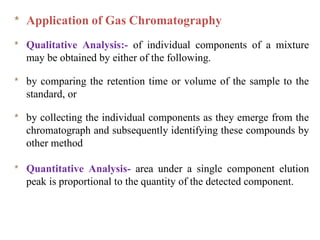
- 26. * Application of Gas Chromatography * Qualitative Analysis:- of individual components of a mixture may be obtained by either of the following. * by comparing the retention time or volume of the sample to the standard, or * by collecting the individual components as they emerge from the chromatograph and subsequently identifying these compounds by other method * Quantitative Analysis- area under a single component elution peak is proportional to the quantity of the detected component.
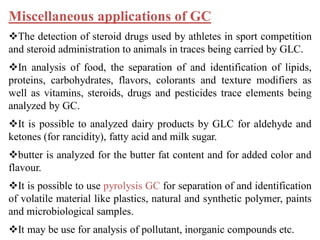
- 27. Miscellaneous applications of GC The detection of steroid drugs used by athletes in sport competition and steroid administration to animals in traces being carried by GLC. In analysis of food, the separation of and identification of lipids, proteins, carbohydrates, flavors, colorants and texture modifiers as well as vitamins, steroids, drugs and pesticides trace elements being analyzed by GC. It is possible to analyzed dairy products by GLC for aldehyde and ketones (for rancidity), fatty acid and milk sugar. butter is analyzed for the butter fat content and for added color and flavour. It is possible to use pyrolysis GC for separation of and identification of volatile material like plastics, natural and synthetic polymer, paints and microbiological samples. It may be use for analysis of pollutant, inorganic compounds etc.
- 28. Thank You !