Hardness of water
- 2. Applied Chemistry LEARNING TARGETS ïą Basics of water ïą Sources of water ïą Impurities in water ïą Effect of impurities ïą Alkalinity of water ïą Hardness of water
- 3. Applied Chemistry ïą Troubleshooting due to hard water Boiler corrosion, causes and prevention Caustic imbrittleness , causes and prevention ïą Softening of water Soda lime process Zeolite Ion exchanger ïą Water treatment for municipal water supply
- 4. Applied Chemistry Basics of water âA clear transparent liquid, perfectly neutral in reaction and devoid of taste or smellâ In the pure state it is composed of oxygen and hydrogen in proportion of 1:2 by volume
- 5. Applied Chemistry Concept of pure water âWater that is free from objectionable color, odor, taste & turbidity is called pure waterâ âPure water which contains adequate amount of dissolved oxygen but no microorganisms and other organic pollutants and is free of microbial and chemical toxins is called wholesome waterâ Pure water Wholesome water
- 6. Applied Chemistry Key H2O Characteristics âĒ Water is clear, transparent, tasteless liquid at ordinary temp. in the pure state. âĒ It is prime constituent of all living organisms. âĒ Water moves easily from one physical state to another, and from one place to another. âĒ Its specific gravity is 1 âĒ It is bad conductor of heat and electricity âĒ It expands on heating and at 1000c it gives invisible vapors at 1 atm. pressure âĒ On cooling it contracts till 40c âĒ It has maximum density at 40c âĒ Further lowering of temperature, it further contracts and solidify at 00c âĒ Water slowly absorbs and releases large quantities of energy. âĒ Water is a superior solvent and solubalise many solute at low or elevated temp âĒ It liberates heat when H2SO4 or HCl is added to it. âĒ NH4Cl, NH4SCN, NaNO2 is added to it.
- 8. Applied Chemistry How much water is available ?
- 9. Applied Chemistry âĒIt is a universal solvent. âĒIt is an efficient transport medium (nutrients) âĒFacilitates thermoregulation in body. âĒHelps in maintenance of blood and plasma volume âcellular osmotic pressure. âĒAssist in secretary and excretory functions of bodyâconstituent of enzyme & hormonal secretions. âĒValuable medium for administration of therapeutics. âĒHelps in propagating useful aquatic flora and fauna. âĒEssential for irrigation, power generation & domestic purpose. Functions of Water
- 10. Applied Chemistry âĒ Industrial growth & development has increased the sewage production. âĒ Increased flow of sewage and organic matter has increased the BOD from 1 mg/liter (for neutral waters) to 300-500 mg/liter (polluted water). âĒ Micro-organisms present in farm and domestic effluents are added to fresh water sources (rivers & lakes). âĒ Dissolved salts can make water hard and unpalatable âĒ Industrial wastes rich in intermediate products & heavy metals, etc are added to river streams. Need for water purification
- 11. Applied Chemistry âĒTo remove color, objectionable odor and taste. âĒTo remove dissolved gases and suspended solids. âĒTo remove suspended and dissolved organic solids. âĒTo remove pathogenic bacteria. âĒTo make water safe for industrial, drinking and domestic purpose. Objectives of Water Purification
- 12. Applied Chemistry Sources Rain water Surface water Sea Water Lakes and ponds Stream and riverGround water Sources of water
- 14. 1. Suspended impurities Clay, sand, Vegetable matter etc. 2. Dissolved impurities Bicarbonate, Chloride, Sulfate of Ca and Mg Dissolved O2, CO2, H2S 3. Colloidal impurities Fe(OH)3, Al(OH)3 4. Biological impurities Impurities in water
- 15. Applied Chemistry Effect of impurities 1. Imparts color 2 Impart odor 3. Impart Taste 4. Impart Turbidity 5. Impart Alkalinity 6. Impart Hardness Minerals
- 16. It is mainly due to 1. Only hydroxide (OH-) 2. Only carbonate (CO3 - -) 3. Only Bicarbonate (HCO3 - ) 4. Both hydroxide (OH-)and carbonate (CO3 - -) 5. Both carbonate (CO3 - -)and bicarbonate (HCO3 - ) 6. But not due to hydroxide (OH-) and (HCO3 - ) Alkalinity of water OH- + HCO3 - H2O + CO2
- 17. Methods of determination of alkalinity of water Water Sample Phenolphthaline Pink color Phenolphthalein end point (colorless) (P) Methyl orange indicator Yellow Methyl orange end point (red color) (M)
- 18. 1. Temporary hardness ï It is due to bicarbonate of calcium and magnesium ï It is removed by boiling Boil soluble In soluble Ca (HCO3)2 CaCO3 + H2O + CO2 Mg (HCO3)2 MgCO3 + H2O + CO2 soluble In soluble Boil Hardness of water
- 19. ï It is due to chloride and sulfate of calcium and magnesium ï It is not removed by boiling 2. Permanent hardness
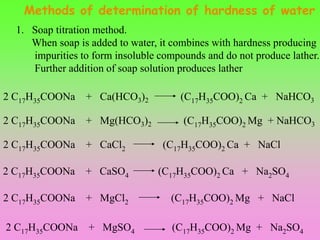
- 20. Methods of determination of hardness of water 1. Soap titration method. When soap is added to water, it combines with hardness producing impurities to form insoluble compounds and do not produce lather. Further addition of soap solution produces lather 2 C17H35COONa + CaSO4 (C17H35COO)2 Ca + Na2SO4 2 C17H35COONa + CaCl2 (C17H35COO)2 Ca + NaCl 2 C17H35COONa + MgSO4 (C17H35COO)2 Mg + Na2SO4 2 C17H35COONa + MgCl2 (C17H35COO)2 Mg + NaCl 2 C17H35COONa + Ca(HCO3)2 (C17H35COO)2 Ca + NaHCO3 2 C17H35COONa + Mg(HCO3)2 (C17H35COO)2 Mg + NaHCO3
- 21. 2. EDTA method Structure of EDTA CH2CH2 NN CH2COOH CH2COOH HOOCCH2 HOOCCH2 CH2CH2 NN OOCH2C CH2COOHCH2COOH CH2COO M Structure of Metal EDTA complex
- 22. Applied Chemistry Units of Hardness Hardness of water is express in CaCO3 equivalent For conversion of any salt into its CaCo3 Equivalent it is multiplied by a multiplication number CaCO3 equivalent of salt = Wt of salt x M. W. of CaCO3 / M. W. salt
Editor's Notes
- #2: J D College of Engineering Nagpur