Health Informatics and implantable medical devices
- 1. FUTURE DIRECTION OF MEDICAL DEVICE LABELING AND WHY THIS IS GOOD FOR INDUSTRY Myron Finseth Medtronic Problems with medical device information HL7 Structured Product Labeling (SPL) format 1
- 2. Problems with medical device labeling • No universal method for patients or clinicians to quickly find device information. – Web site designs are company-specific. No consistent organization or search mechanisms. – No consistent document type exists for device documentation. Table of contents are company-specific and change from device to device. • No standard process for updating documentation throughout the lifecycle. – No industry-wide mechanism for patients, clinicians, and physicians to know that they have the most up-to-date product information. • Patients and clinicians rely heavily on company call centers for basic information. – No sure way to know if you have the right manual for a specific device model. 2
- 3. FDA initiatives to address labeling problems Outcomes from the FDA Public Workshop (May 2013) • Possible implementation of standardized content and format of medical device labeling (1). • List of required sections in medical device labeling. • Possible implementation of a centralized repository for medical device labeling, similar to drugs and biologics. • Use of the UDI to get the most recent labeling for a specific device model. • Possible use of a standardized xml format, such as the SPL standard, which is used to format UDI information. 3 (1) Source: Mary Brady, Senior Policy Analyst, CDRH 510(k) Implementation Meeting on Device Labeling. April 7, 2011.
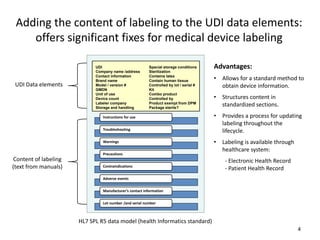
- 4. Adding the content of labeling to the UDI data elements: offers significant fixes for medical device labeling 4 UDI Data elements Content of labeling (text from manuals) Instructions for use Troubleshooting Warnings Precautions Contraindications Adverse events Manufacturer’s contact information Lot number /and serial number Special storage conditions Sterilization Contains latex Contain human tissue Controlled by lot / serial # Kit Combo product Controlled by Product exempt from DPM Package sterile? UDI Company name /address Contact information Brand name Model / version # GMDN Unit of use Device count Labeler company Storage and handling Advantages: • Allows for a standard method to obtain device information. • Structures content in standardized sections. • Provides a process for updating labeling throughout the lifecycle. • Labeling is available through healthcare system: - Electronic Health Record - Patient Health Record HL7 SPL R5 data model (health Informatics standard)
- 5. Flow of structured, regulated product information (labeling for medical devices, drugs, biologics, or combination products) 5
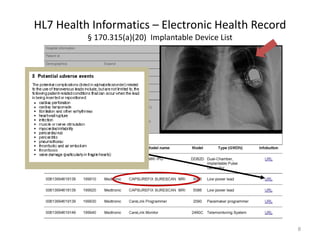
- 6. Electronic Health Records (EHR) ONC Certification Criteria: § 170.315(a)(20) Implantable Device List 1. Record a minimum set of data elements for each UDI in a patient's implantable device list. 2. Accept electronic UDI data via automatic identification and data capture (AIDC) or other assistive technologies used in health care systems (e.g., bar code scanners and radio frequency identification). 3. Use the device identifier portion of the UDI to obtain and incorporate GUDID device identification attributes in the patient’s implantable device list. 6
- 7. HL7 Health Informatics – Electronic Health Record § 170.315(a)(20) Implantable Device List 7
- 8. HL7 Health Informatics – Electronic Health Record § 170.315(a)(20) Implantable Device List 8
- 9. HL7 Health Informatics – Electronic Health Record § 170.315(a)(20) Implantable Device List 9 US Meaningful Use Goals for 2017 -2020 – All patients to have a unique EHR used by clinics and hospitals. – EHR functions as the clinician’s desktop, where information is provided and entered. – EHRs to include an Implantable Device List (CFR 170.315(a)(20) – Implantable Device List to include basic set of UDI data elements with the capability of referencing in all data elements from FDA UDI Database.