Hematopathology Lecture.pdf
- 1. Laboratory Data Interpretation- Haematopatholohy -I Department of Pathology College of Medicine University of Sulaimani August 2022 1
- 2. ’Ƭ Anticoagulants in use ’Ƭ Collection artifacts ’Ƭ Blood samples for cross match ’Ƭ RBCs indices in CBC ’Ƭ Questions ’Ƭ Take home message Topics 2
- 4. ’Ƭ Difficult venipuncture ’Ƭ Low sample volume ’Ƭ Inappropriate mixing with anticoagulant ’Ƭ Dilution with fluids ’Ƭ Rough handling (Mechanical hemolysis) Collection artifacts 4
- 5. ’Ƭ Slow or traumatic venipuncture (poking around a lot for the vein, exiting the vein during sample withdrawal) can precipitate platelet clumping and induce small microclots within the sample or even clotting of the sample and may plug the tubing of the hematology analyzer. Difficult venipuncture 5
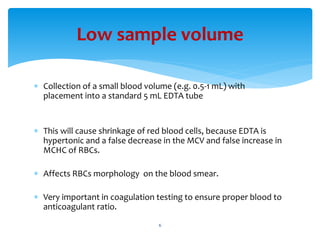
- 6. ’Ƭ Collection of a small blood volume (e.g. 0.5-1 mL) with placement into a standard 5 mL EDTA tube ’Ƭ This will cause shrinkage of red blood cells, because EDTA is hypertonic and a false decrease in the MCV and false increase in MCHC of RBCs. ’Ƭ Affects RBCs morphology on the blood smear. ’Ƭ Very important in coagulation testing to ensure proper blood to anticoagulant ratio. Low sample volume 6
- 7. ’Ƭ This which may not be visible to the naked eye (microclots). Inappropriate mixing with anticoagulant 7
- 8. ’Ƭ Collection of blood for hematologic testing through an in-dwelling catheter is not optimal, but may be necessary in critically ill patients that are being frequently sampled for monitoring of changes. Dilution with fluids 8
- 9. ’Ƭ Shaking of blood tubes, forcing blood through needles, vigorous expulsion into tubes, can cause shearing of red blood cells (hemolysis) and platelet clumping. ’Ƭ Mechanical hemolysis may be also caused by the use of small- gauge needles, trauma to a vein. Rough handling 9
- 11. Citrate Tubes 11
- 12. ’Ƭ Blood Samples for Cross Match 12
- 13. Sample Collection Tubes ’Ƭ Patient samples for compatibility testing may be serum or plasma. ’Ƭ Plasma samples are preferred. ’Ƭ The collection tubes are spray-coated with K2EDTA and provide anticoagulated blood with plasma and red cells for testing. 13
- 14. Age of Sample ’Ƭ Samples should be collected no more than 3 days of the scheduled transfusion ?Why ’Ƭ Patient samples and a segment from the donor unit used for cross matching must be stored for at least 7 days after transfusion . 14
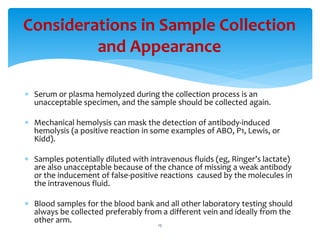
- 15. Considerations in Sample Collection and Appearance ’Ƭ Serum or plasma hemolyzed during the collection process is an unacceptable specimen, and the sample should be collected again. ’Ƭ Mechanical hemolysis can mask the detection of antibody-induced hemolysis (a positive reaction in some examples of ABO, P1, Lewis, or Kidd). ’Ƭ Samples potentially diluted with intravenous fluids (eg, RingerŌĆÖs lactate) are also unacceptable because of the chance of missing a weak antibody or the inducement of false-positive reactions caused by the molecules in the intravenous fluid. ’Ƭ Blood samples for the blood bank and all other laboratory testing should always be collected preferably from a different vein and ideally from the other arm. 15
- 16. ’ƬRed Blood Cells indices 16
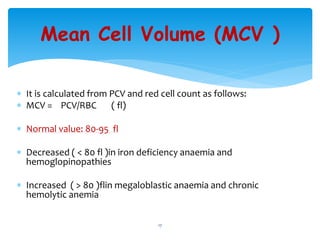
- 17. Mean Cell Volume (MCV ) ’Ƭ It is calculated from PCV and red cell count as follows: ’Ƭ MCV = PCV/RBC ( fl) ’Ƭ Normal value: 80-95 fl ’Ƭ Decreased ( < 80 fl )in iron deficiency anaemia and hemoglopinopathies ’Ƭ Increased ( > 80 )flin megaloblastic anaemia and chronic hemolytic anemia 17
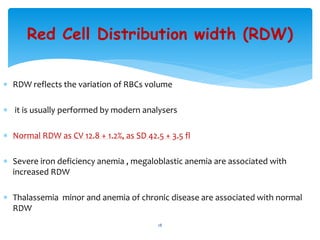
- 18. Red Cell Distribution width (RDW) ’Ƭ RDW reflects the variation of RBCs volume ’Ƭ it is usually performed by modern analysers ’Ƭ Normal RDW as CV 12.8 + 1.2%, as SD 42.5 + 3.5 fl ’Ƭ Severe iron deficiency anemia , megaloblastic anemia are associated with increased RDW ’Ƭ Thalassemia minor and anemia of chronic disease are associated with normal RDW 18
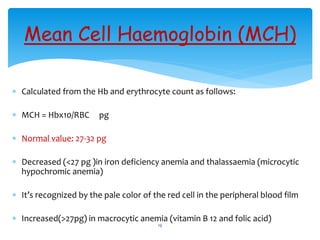
- 19. Mean Cell Haemoglobin (MCH) ’Ƭ Calculated from the Hb and erythrocyte count as follows: ’Ƭ MCH = Hbx10/RBC pg ’Ƭ Normal value: 27-32 pg ’Ƭ Decreased (<27 pg )in iron deficiency anemia and thalassaemia (microcytic hypochromic anemia) ’Ƭ ItŌĆÖs recognized by the pale color of the red cell in the peripheral blood film ’Ƭ Increased(>27pg) in macrocytic anemia (vitamin B 12 and folic acid) 19
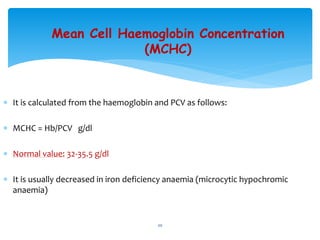
- 20. Mean Cell Haemoglobin Concentration (MCHC) ’Ƭ It is calculated from the haemoglobin and PCV as follows: ’Ƭ MCHC = Hb/PCV g/dl ’Ƭ Normal value: 32-35.5 g/dl ’Ƭ It is usually decreased in iron deficiency anaemia (microcytic hypochromic anaemia) 20
- 21. 21
- 22. Anemia Microcytic Macrocytic Normocytic Classification of anaemias using MCV 22
- 23. Iron Deficiency Anaemia Anaemia of Chronic Disorder Lead Poisoning Thalassaemia Sideroblastic Anaemia Microcytic Anaemia MCV<80 fl 23
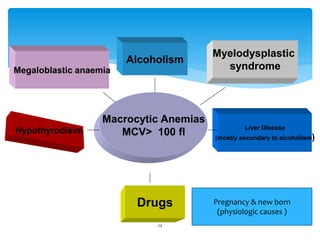
- 24. Megaloblastic anaemia Hypothyrodisim Liver Disease (mostly secondary to alcoholism) Drugs Alcoholism Myelodysplastic syndrome Macrocytic Anemias MCV> 100 fl Pregnancy & new born (physiologic causes ) 24
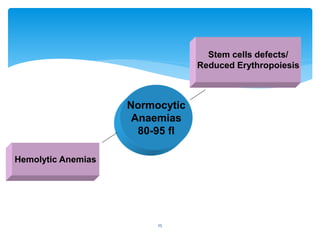
- 25. Normocytic Anaemias 80-95 fl Stem cells defects/ Reduced Erythropoiesis Hemolytic Anemias 25
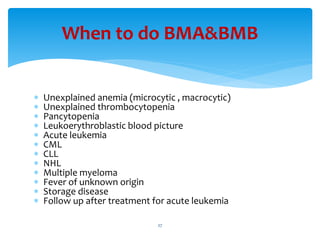
- 26. ’Ƭ When to send for blood film ( CBP) ’Ƭ When to send for Hb-electrophoresis ’Ƭ What should I write in haematology request( shortly all relevant information , including recent transfusion when present) ’Ƭ Samples transport &storage ’Ƭ When to do BMA&BMB Frequently asked questions 26
- 27. ’Ƭ Unexplained anemia (microcytic , macrocytic) ’Ƭ Unexplained thrombocytopenia ’Ƭ Pancytopenia ’Ƭ Leukoerythroblastic blood picture ’Ƭ Acute leukemia ’Ƭ CML ’Ƭ CLL ’Ƭ NHL ’Ƭ Multiple myeloma ’Ƭ Fever of unknown origin ’Ƭ Storage disease ’Ƭ Follow up after treatment for acute leukemia When to do BMA&BMB 27
- 28. ’Ƭ Always correlate the laboratory results with clinical data and interpret accordingly . ’Ƭ Remember possible causes for laboratory tests errors. Take home message 28
- 29. Thank you 29