Hydroxy appatite and its interaction with milk
- 1. welcome
- 2. Presentation on: Hydroxyappatite Presented by: M. Archana 1st Yr Mtech Dairy Chemistry
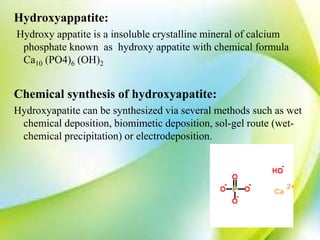
- 3. Hydroxyappatite: Hydroxy appatite is a insoluble crystalline mineral of calcium phosphate known as hydroxy appatite with chemical formula Ca10 (PO4)6 (OH)2 Chemical synthesis of hydroxyapatite: Hydroxyapatite can be synthesized via several methods such as wet chemical deposition, biomimetic deposition, sol-gel route (wet- chemical precipitation) or electrodeposition.
- 4. Interaction of hydroxyappatite with milk proteins and minerals: ď‚— Bone health is an issue of growing importance with osteoporosis on the increase largely due to and ageing global population(Gurr, etal., 1999). ď‚— Dietary calcium is a crucial factor in maintaining the bone health and combating osteoporosis(Heney etal., 2000). ď‚— Therefore calcium fortification is a growing trend in food industries this is especially true for milk and dairy products as they all naturally rich in calcium and already wellknown for their benefits for bone, health.(Augustin and williams, 2002)
- 5. ď‚— Milk in its natural state contains approximately 120mg of Calcium per 100g (Lucey and Horne, 2009) to fortify milk and dairy products further with calcium, calcium salts such as calcium chloride, calcium phosphate, calcium citrate need to be added to milk.(Grandison and Lewis, 2010; Singh etal., 2007). ď‚— Selecting a good source of calcium for fortification requires a good understanding of its physical and chemical properties and how it interacts with milk components(Philippe, Gucheron, 20003). ď‚— UHT treatment increases the shelf life of liquid milk by rendering them sterile and bacteriologically stable for several months at ambient temperature (Datta and Deeth, 2001)
- 6. ď‚— UHT treatment of unfortified milk is rarely a problem as it can withstand such heat treatment without any major issue, however there are a number of practical issues related to the processing and formulation of calcium fortified UHT milk, that makes it challenging to maintain stability of product over its intended shelf life . ď‚— If soluble calcium salts such as calcium chloride, calcium lactate are used to fortify milk with calcium their addition can lead to protein aggregation and heat instability during the UHT treatment. ď‚— The mineral equilibrium is disrupted by the soluble salts there by reducing the electrostatic repulsion between the micelles and leading to their aggregation on heat treatment.
- 7. ď‚— In commercial UHT calcium fortified milk, insoluble calcium salts such as Tricalcium phosphate, Hydroxyappatite and calcium carbonate are usually used as calcium fortificants. Since they are considered to be chemically unreactive in milk therefore not causing heat instability. ď‚— But the use of such calcium (insoluble) forms causes shelflife instability, such as phase separation and sedimentation occurs(Gucheron, 2003). ď‚— It is well known that HA does interact with proteins, as proteins adsorbed strongly onto HA in a wide range of biological applications such as bioceramics material, chromatography(Kawasaki, 1991 and Zhang, 2012).
- 8.  Milk protein adsorption on to HA particles may occur in calcium fortified milks, and could potentially modify the colloidal stability of the HA particles in the product or the stability of milk protein fraction.  The adsorption behavior of αs1-CN, β-CN, k-CN, α-La and β- Lg on HA was characterized by determination of adsorbed protein levels and surface charge of HA.
- 15. Thank you