Ict Article Gct Info Mar07
- 1. ict Global Outsourcing 400 A Home for Trials: the Bloc Reborn 300 Eastern Europe â a place for experienced sites, motivated patients and a history of success. Richard Leach of RCT Global, LLC, takes a closer look at Russia, the largest, most populated 200 member of this research centric region Richard Leach is Vice President and Head of Business Development for RCT-Global LLC, a CRO with offices in Russia, Bulgaria and the US that uses a global network of regional providers to support clinical 100 research worldwide. Prior to joining RCT-Global in 2003, Richard was Director of Business Development for PharmaNet, responsible for developing corporate relationships with key international accounts. With over 15 yearsâ experience in the pharmaceutical industry, he has had the pleasure of working with companies offering international services as diverse as centralised laboratory and electrocardiogram (ECG) monitoring to regulatory support and project management. Prior to his position at PharmaNet, Richard held numerous positions with Covance Labs, Premier Research, and Quintiles Late Stage Development. Eastern Europe continues to be one of the most active regions in both internal BMS audits and FDA inspections: âOverall the world for clinical research. Since the early to mid 1990s, deficiencies were found to be far less common in Central and pharmaceutical companies and the CROs that support them Eastern Europe than in Western Europe. Up to 10 per cent of have run thousands of clinical trials, invested millions of BMSâ global data comes from Central and Eastern Europe. On dollars, and opened or expanded offices throughout the region individual studies, sometimes as much as 50 per cent of the data to accommodate unprecedented growth. Why has growth been comes from this regionâ (2). so dramatic and what continues to make Eastern Europe the second highest patient recruiting region in the world for ENROLMENT ENVIRONMENT industry sponsored clinical trials, after the US (1)? In order to consider this question we need to first reflect on the history of The reasons for patients within Eastern Europe to participate in this phenomenon, and then examine how these changes affect clinical research are as varied as in other parts of the world. the region today. Table 1: Population Overview of Region RESEARCH PIONEERS Population (rounded to Country the nearest thousand) Research organisations focused on this region initially to Belarus 9,755,000 supplement patient enrolment in trials where recruitment was not Bosnia and Herzegovina 4,499,000 meeting expectations. With a population of almost 325 million, and with large centralised health facilities offering deep pools of Bulgaria 7,726,000 potential patients, the attractiveness of the region was justified. Croatia 5,025,000 Once trials were introduced to the region, these early sponsors Czech Republic 10,265,000 and research organisations discovered site productivity, across all Estonia 1,330,000 therapeutic areas, was consistently higher than either Western Hungary 10,076,000 Europe or the US. As the region developed and more countries Latvia 2,307,000 created central ethics committees, formed solid government Lithuania 3,412,000 oversight and adopted legislation in support of ICH-GCP, more investment poured into the region. However, once the early Montenegro 631,000 research pioneers successfully began submitting data generated Poland 38,128,000 from investigator sites and patients across this region, the level Republic of Macedonia 2,034,000 of interest within the industry soared. Romania 22,304,000 Russia 142,400,000 Many of these organisations are now coming to this region Serbia 9,779,000 because of the bulk of their international patient databases. Slovakia 5,401,000 What they have found is that the regulatory oversight and the Slovenia 2,004,000 commitment to GCP is of the highest quality. According to Cezary Statuch, Executive Director of Global Development Ukraine 46,481,000 Operations for Bristol-Myers Squibb, the quality of data coming Total Population 323,557,000 from Central/Eastern European sites is superb, as indicated by 36 ICT www.samedanltd.com
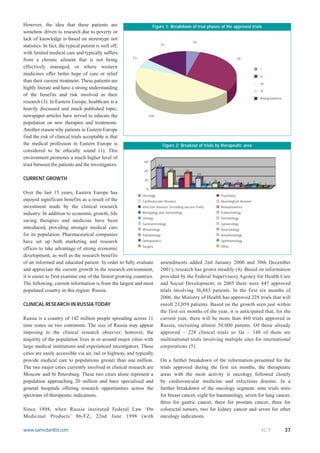
- 2. However, the idea that these patients are Figure 1: Breakdown of trial phases of the approved trials somehow driven to research due to poverty or lack of knowledge is based on stereotype not 10 statistics. In fact, the typical patient is well off, 31 with limited medical care and typically suffers 13 70 from a chronic ailment that is not being effectively managed, or where western I medicines offer better hope of cure or relief II than their current treatment. These patients are III highly literate and have a strong understanding IV of the benefits and risk involved in their Bioequivalence research (3). In Eastern Europe, healthcare is a heavily discussed and much published topic; newspaper articles have served to educate the 104 population on new therapies and treatments. Another reason why patients in Eastern Europe find the risk of clinical trials acceptable is that the medical profession in Eastern Europe is Figure 2: Breakout of trials by therapeutic area considered to be ethically sound (1). This environment promotes a much higher level of 60 trust between the patients and the investigators. 42 34 31 40 20 20 18 CURRENT GROWTH 20 7 9 7 7 6 5 4 5 1 3 2 4 2 1 0 Over the last 15 years, Eastern Europe has Oncology Psychiatry enjoyed significant benefits as a result of the Cardiovascular diseases Neurological diseases investment made by the clinical research Infection diseases (including vaccine trials) Bioequivalence industry. In addition to economic growth, life Allergology and immunology Endocrinology Urology Dermatology saving therapies and medicine have been Gastroenterology Gynaecology introduced, providing stronger medical care Rheumology Haematology for its population. Pharmaceutical companies Pulmonology Anesthesiology have set up both marketing and research Orthopaedics Ophthalmology Surgery Other offices to take advantage of strong economic development, as well as the research benefits of an informed and educated patient. In order to fully evaluate amendments added 2nd January 2000 and 30th December and appreciate the current growth in the research environment, 2001), research has grown steadily (4). Based on information it is easier to first examine one of the fastest growing countries. provided by the Federal Supervisory Agency for Health Care The following, current information is from the largest and most and Social Development, in 2005 there were 447 approved populated country in this region: Russia. trials involving 36,883 patients. In the first six months of 2006, the Ministry of Health has approved 228 trials that will CLINICAL RESEARCH IN RUSSIA TODAY enroll 23,059 patients. Based on the growth seen just within the first six months of the year, it is anticipated that, for the Russia is a country of 142 million people spreading across 11 current year, there will be more than 460 trials approved in time zones on two continents. The size of Russia may appear Russia, recruiting almost 50,000 patents. Of those already imposing to the clinical research observer; however, the approved â 228 clinical trials so far â 148 of them are majority of the population lives in or around major cities with multinational trials involving multiple sites for international large medical institutions and experienced investigators. These corporations (5). cities are easily accessible via air, rail or highway, and typically provide medical care to populations greater than one million. On a further breakdown of the information presented for the The two major cities currently involved in clinical research are trials approved during the first six months, the therapeutic Moscow and St Petersburg. These two cities alone represent a areas with the most activity is oncology followed closely population approaching 20 million and have specialised and by cardiovascular medicine and infectious disease. In a general hospitals offering research opportunities across the further breakdown of the oncology segment: nine trials were spectrum of therapeutic indications. for breast cancer, eight for haematology, seven for lung cancer, three for gastric cancer, three for prostate cancer, three for Since 1998, when Russia instituted Federal Law âOn colorectal tumors, two for kidney cancer and seven for other Medicinal Productsâ 86-FZ, 22nd June 1998 (with oncology indications. www.samedanltd.com ICT 37
- 3. Current growth in Russia is driven by many of the same factors best work within the current system in order to smoothly handle that push clinical research in other Eastern European countries. shipments of both study drug and laboratory specimens. Having The history of successful trial completion with high data quality a strong vested interest in the sponsorsâ success ensures has established strong sponsor confidence. There are many continued growth for their respective organisations and drivers that have helped to build confidence in this region; the increases interest in their country across the industry. following represent a selection of those that have played a part. CONCLUSION Site Experience and Commitment Every site involved in clinical research has to be included on an Eastern Europe may be the second highest patient recruiting approved list of sites, and submitted to the MoH with the region of the world in industry-sponsored clinical trials, but application to perform the trial, ensuring that only experienced there is still tremendous room for growth and investment. This investigators can participate. In order to be included on the list, region offers a patient population equal to that of the US, yet the investigator must have two yearsâ clinical trials experience currently those with the opportunity to participate in studies is and have received training in Good Clinical Practices. In far smaller. As a potential market for new medicines, the addition, many of the sites are located in large medical opportunity will only increase as each of these nations institutions in cities with good public transportation, allowing continues to strengthen their economy and develop an for excellent patient access. Many of these sites, being located increasing interest in new medicines and treatments. Russia in large medical institutions, have databases of patients with alone has a population of 142 million, yet at present less than differing pathology that can be used to accelerate enrolment 100,000 patients participate in research over the course of a during trial initiation and throughout the trial. In addition to year. Eastern Europe represents an unprecedented opportunity their experience and access to patients, investigators in Eastern for pharmaceutical and biotech organisations developing new Europe are experienced in working within the bureaucracy of medicines. The infrastructure is sound, the quality is proven and state run healthcare, making them familiar with the large the potential is only now beginning to be fully realised. N amounts of documentation and accustomed to processing paperwork accurately and completely. The author can be contacted at richard.leach@cro-rct.ru Motivated and Knowledgeable Patient Population References As discussed already, the patient population in Eastern Europe is educated and knowledgeable about clinical research. As a 1. Tassignon JP, BioEthics in Eastern Europe, GOR, Vol 7, result of the current limitations of a state sponsored medical No 1, pp65-68, Spring 2005 system in transition, patients are motivated to participate in clinical research. They see clinical trials as an opportunity to 2. Borfitz D, Expanding Opportunities in Central receive the highest level of medical care, while having access and Eastern Europe, CenterWatch Monthly, Volume 11, to new treatments that previously were unavailable to them. Issue 4, p5, April 2004 Due to protocol requirements, they often have more time with their doctor than usual, and the diagnostic testing required to 3. Platonov P, Clinical Trials in Russia and Eastern qualify patients frequently exceeds the standard practice of Europe: Recruitment Quality, International Journal of the region. As a result, the Eastern European patient tends to Clinical Pharmacology and Therapeutics, Vol 4, No7, be more compliant and less prone to leave a trial prior to pp277-280, 2003 study completion. 4. Russia first adopted laws for governing clinical research in Experienced and Dedicated Service Providers 1998 with the Federal Law, âOn Medicinal Productsâ, 86-FZ The atmosphere in Russia, as in Eastern Europe, is right for dated 22nd June 1998 (with the last amendment dated clinical research, due to experienced and committed sites, and 29th December 2004). In 1999 they incorporated ICH GCP motivated, educated and knowledgeable patients. However there guidelines into industrial standard with the passing of OST is another factor to consider when working in this region that 42-511-99 Rules of Clinical Trials Conduct in Russian completes this picture: the service providers â regional CROs â Federation and further defined the law in 2003 with the working throughout Eastern Europe are proud of their role in Decree of the Ministry of Health of RF #266 dated 19th research and committed to the satisfaction of their customers. June 2003, titled About Approving of Clinical Practice These service providers understand the importance of success to Rules in Russian Federation. Now the National Standard their organisation and the country in which they work. They of Russian Federation GOST R 52379-2005 âGood Clinical provide highly educated CRAs, often MDs, to interact with the Practiceâ is in force. This standard is completely identical sites providing strong monitoring support. These CROs are to ICH GCP. experienced in working with the numerous regulatory groups, and frequently have personal relationships that help to facilitate 5. The Federal Supervisory Agency for Health Care and Social approval and maintain a high level of communications. Local Development site address is http://www.roszdravnadzor.ru/ providers support import and export activities, and know how to medcontrol/clinic/ind?year=2006 38 ICT www.samedanltd.com
- 4. General Information The recent growth in pharmaceutical development in the Russian Federation, Bulgaria, Romania and the Ukraine has attracted the attention of drug development companies all over the world. Pharmaceutical and Biotech companies along with numerous international clinical research organizations have seen the value and have invested heavily. Everyday new offices are opening and trials are being initiated. Though this growth can be attributed to many things from the globalization of research to increasingly friendly regulatory environments one key element continues to drive interest, Patient Availability. The Russian Federation, Bulgaria, Romania and the Ukraine represent a population in excess of 200 million people. In major cities like Moscow, St. Petersburg, Novosibirsk, Sofia, Kiev and Odessa there are numerous hospitals and scientific research centers that are highly-specialized offering hundreds of beds. Many of these centers are pathology specific and attract patients from the local area (over 70% of the population live in and around major population centers). City - Country Population Global Clinical Trials, LLC (GCT) is a Regional CRO with itâs headquarters located in Princeton, NJ and offices in Russia, Moscow â Russia 10,000,000 Bulgaria, Romania and the Ukraine performing clinical research St. Petersburg - services across Eastern Europe. GCT is experienced supporting 4,500,000 Russia trials across all phases of research and therapeutic areas; working Kiev - Ukraine 3,000,000 with international clients from across the globe. Whether you are looking for full clinical support to include site selection, monitoring, Bucharest, Romania 2,100,000 project management, Regulatory, import/export, drug storage, and Sofia - Bulgaria 1,200,000 medical writing; or only interested in an individual service to All City populations have been rounded to the address an urgent need, you can count on GCT to handle your nearest 100,000 requirements professionally and completely. We offer a strong understanding of both local and international regulations as well as access to 1,000s of qualified sites in numerous therapeutic Church on the Blood. areas. The GCT data base identifies over 2,800 experienced sites St. Petersburg, Russia throughout the region by indication and experience. Our pre-qualification process includes a review of ICH/GCP as well as local law for each site, to determine if additional training is required. Our monitors and project managers are all certified clinicians experienced in clinical research. Their relationship with each of their sites is strong and based on mutual respect. As an organization, GCT maintains strong ongoing relationships with the investigators and officials in the Ministry of Health. These relationships help us to develop reliable feasibilities and stay abreast of the changes in governmental regulations and their impact on clinical research. Our expanded logistical services helps ensure smooth and timely receipt of study materials and can also coordinate the shipment and storage of laboratory and PK samples. As your âIn Country Advocateâ, GCT will represent your organization with the kind of professionalism and attention to detail you would expect from your own team, except with the regional knowledge and local experience that can only come from years of working within the Region. Please call us and discover the âGCT Experienceâ for yourself, you wonât be disappointed. St. Petersburg, Russia Contact: Richard Leach Moscow, Russia Sofia, Bulgaria Phone: (609) 731-2225 Kiev, Ukraine Bucharest, Romania Princeton, USA