Interhalogens pseudohalogens
- 1. • PRESENTATIO N GROUP MEMBERS 1. Ayesha Arshad 2. Khadija Akram 3. Adsa Khalid 4. Maryam Sarfraz 5. Sana Naseem 1
- 3. InterHalogens : “ Halogen combine with other halogen atom to form binary covalent compounds called Interhalogens “ 3
- 4. Features of INTERHALOGENS :  They are most Electronegative  AB type compounds ( AB,AB3,AB5, AB7) are present.  A is less Electronegative than B  A never F because ( F have high electronegative value and never show +ve oxidation state )  Central Atom has large Atomic Number  If Greater Electronegativity Difference Than greater will be the No of bonds formed 4
- 5.  Types : There are four types of Interhalogens which are : 1 AB type Interhalogens 2 AB3 type Interhalogens 3 AB5 type Interhalogens 4 AB7 type Interhalogens 5
- 6. AB type : ÔÇõ Example : ICL is the example of AB type Interhalogens. Preparation : It is prepared by adding Iodine to liquid Chlorine and keeping the mixture at 30 to 35 temperatures for 24 hours I2 + Cl2 2ICL 6
- 7. AB type : 7
- 8. Properties and Structure : 8
- 9. AB3 Type Interhalogen : 9
- 10. Properties of AB3 : 10
- 11. Structure of AB3 ( ICI3 ) : 11
- 12. AB5 Type : 12
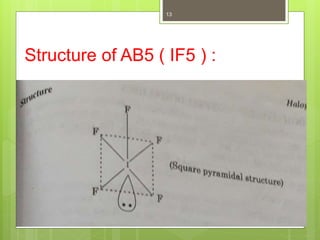
- 13. Structure of AB5 ( IF5 ) : 13
- 14. AB7 Type : 14
- 17. Preparation of Pseudo Halogens : 17
- 18. PROPERTIES: Similarities Between Pseudo halogen And Halogens:  Both react with Alkalis.  Both form Dimer Compounds.  Both shows properties of Addition compounds.  Both form complex with Transition metals. 18