introduction to bioactive glass
- 1. Literature Review Bioactive Materials by Wanpeng Cao & Larry L. Hench Sept 20th 2012 By : Leon Valentino Advisor : Prof. Shao-Ju Shih
- 2. Main Objective • To Study about what is:  Tissue attachment  General Theory of Biomaterials  Bioactivity  Bioactive Ceramics  Mechanism of Bioactive Bonding  Bioactive Coating and Composites Nano Materials and Grain Boundary Engineering Lab 2
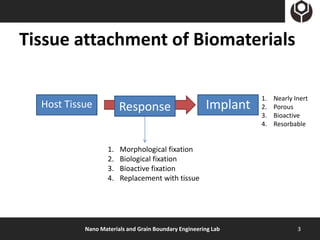
- 3. Tissue attachment of Biomaterials 1. Nearly Inert Host Tissue Response Implant 2. Porous 3. Bioactive 4. Resorbable 1. Morphological fixation 2. Biological fixation 3. Bioactive fixation 4. Replacement with tissue Nano Materials and Grain Boundary Engineering Lab 3
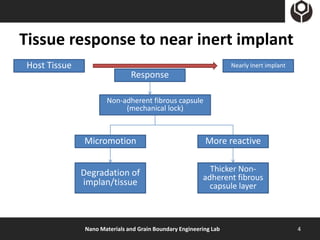
- 4. Tissue response to near inert implant Host Tissue Nearly Inert implant Response Non-adherent fibrous capsule (mechanical lock) Micromotion More reactive Degradation of Thicker Non- adherent fibrous implan/tissue capsule layer Nano Materials and Grain Boundary Engineering Lab 4
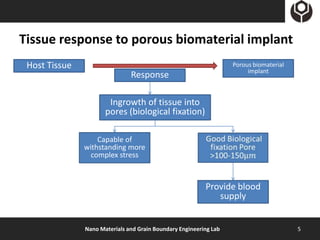
- 5. Tissue response to porous biomaterial implant Host Tissue Porous biomaterial implant Response Ingrowth of tissue into pores (biological fixation) Capable of withstanding more complex stress Provide blood supply Nano Materials and Grain Boundary Engineering Lab 5
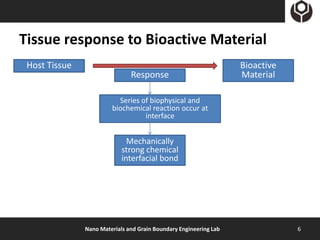
- 6. Tissue response to Bioactive Material Host Tissue Bioactive Response Material Series of biophysical and biochemical reaction occur at interface Mechanically strong chemical interfacial bond Nano Materials and Grain Boundary Engineering Lab 6
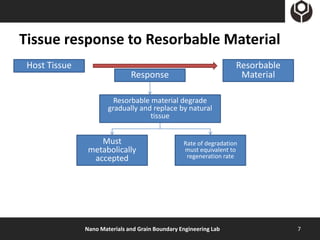
- 7. Tissue response to Resorbable Material Host Tissue Resorbable Response Material Resorbable material degrade gradually and replace by natural tissue Must Rate of degradation metabolically must equivalent to accepted regeneration rate Nano Materials and Grain Boundary Engineering Lab 7
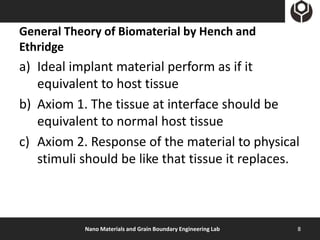
- 8. General Theory of Biomaterial by Hench and Ethridge a) Ideal implant material perform as if it equivalent to host tissue b) Axiom 1. The tissue at interface should be equivalent to normal host tissue c) Axiom 2. Response of the material to physical stimuli should be like that tissue it replaces. Nano Materials and Grain Boundary Engineering Lab 8
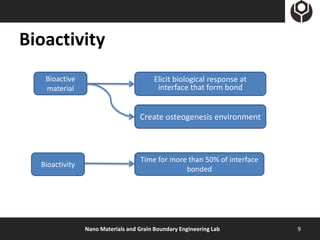
- 9. Bioactivity Bioactive Elicit biological response at material interface that form bond Create osteogenesis environment Time for more than 50% of interface Bioactivity bonded Nano Materials and Grain Boundary Engineering Lab 9
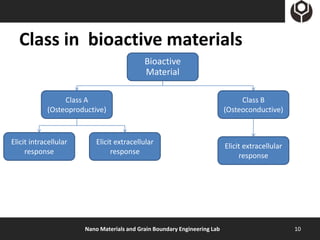
- 10. Class in bioactive materials Bioactive Material Class A Class B (Osteoproductive) (Osteoconductive) Elicit intracellular Elicit extracellular Elicit extracellular response response response Nano Materials and Grain Boundary Engineering Lab 10
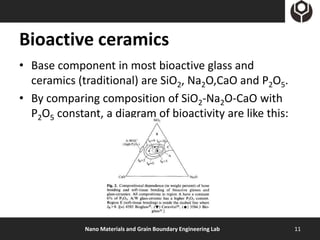
- 11. Bioactive ceramics • Base component in most bioactive glass and ceramics (traditional) are SiO2, Na2O,CaO and P2O5. • By comparing composition of SiO2-Na2O-CaO with P2O5 constant, a diagram of bioactivity are like this: Nano Materials and Grain Boundary Engineering Lab 11
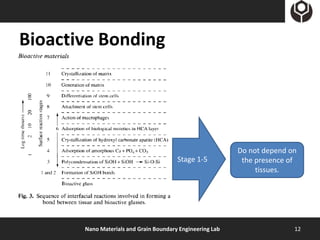
- 12. Bioactive Bonding Do not depend on Stage 1-5 the presence of tissues. Nano Materials and Grain Boundary Engineering Lab 12
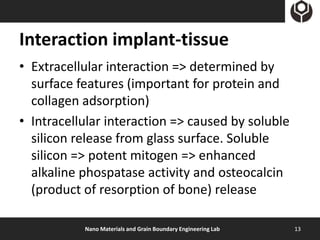
- 13. Interaction implant-tissue • Extracellular interaction => determined by surface features (important for protein and collagen adsorption) • Intracellular interaction => caused by soluble silicon release from glass surface. Soluble silicon => potent mitogen => enhanced alkaline phospatase activity and osteocalcin (product of resorption of bone) release Nano Materials and Grain Boundary Engineering Lab 13
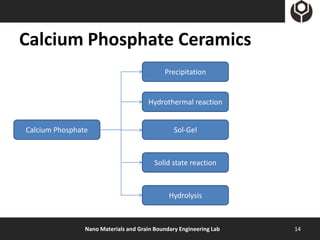
- 14. Calcium Phosphate Ceramics Precipitation Hydrothermal reaction Calcium Phosphate Sol-Gel Solid state reaction Hydrolysis Nano Materials and Grain Boundary Engineering Lab 14
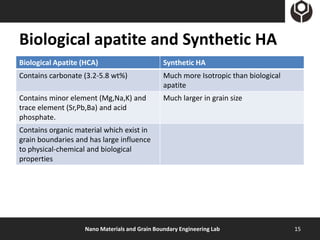
- 15. Biological apatite and Synthetic HA Biological Apatite (HCA) Synthetic HA Contains carbonate (3.2-5.8 wt%) Much more Isotropic than biological apatite Contains minor element (Mg,Na,K) and Much larger in grain size trace element (Sr,Pb,Ba) and acid phosphate. Contains organic material which exist in grain boundaries and has large influence to physical-chemical and biological properties Nano Materials and Grain Boundary Engineering Lab 15
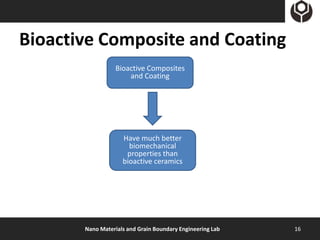
- 16. Bioactive Composite and Coating Bioactive Composites and Coating Have much better biomechanical properties than bioactive ceramics Nano Materials and Grain Boundary Engineering Lab 16
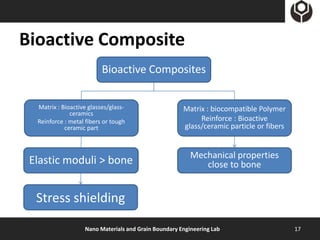
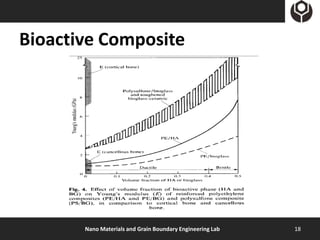
- 17. Bioactive Composite Bioactive Composites Matrix : Bioactive glasses/glass- Matrix : biocompatible Polymer ceramics Reinforce : metal fibers or tough Reinforce : Bioactive ceramic part glass/ceramic particle or fibers Mechanical properties Elastic moduli > bone close to bone Stress shielding Nano Materials and Grain Boundary Engineering Lab 17
- 18. Bioactive Composite Nano Materials and Grain Boundary Engineering Lab 18
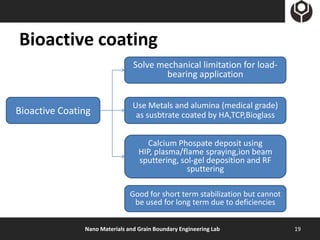
- 19. Bioactive coating Solve mechanical limitation for load- bearing application Use Metals and alumina (medical grade) Bioactive Coating as susbtrate coated by HA,TCP,Bioglass Calcium Phospate deposit using HIP, plasma/flame spraying,ion beam sputtering, sol-gel deposition and RF sputtering Good for short term stabilization but cannot be used for long term due to deficiencies Nano Materials and Grain Boundary Engineering Lab 19
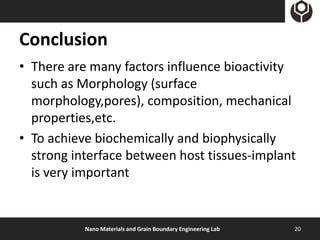
- 20. Conclusion • There are many factors influence bioactivity such as Morphology (surface morphology,pores), composition, mechanical properties,etc. • To achieve biochemically and biophysically strong interface between host tissues-implant is very important Nano Materials and Grain Boundary Engineering Lab 20
- 21. Future Work • Produce MBG using Sol-Gel methode for temp 400 C and 600C Nano Materials and Grain Boundary Engineering Lab 21
- 22. Nano Materials and Grain Boundary Engineering Lab 22










![Natural and synthetic polymers in medicine ppt [Autosaved].pptx](https://cdn.slidesharecdn.com/ss_thumbnails/naturalandsyntheticpolymersinmedicinepptautosaved-220515192625-27c424c5-thumbnail.jpg?width=560&fit=bounds)







































