Introduction to crystallography and x ray diffraction theory
- 1. Effect of Mineral's Crystal Structure on X-Ray Diffraction Test Senior Chemical Engineer Raghd Muhi Al-Deen Jassim
- 3. Diffraction occurs when light is scattered by a periodic array with long-range order, producing constructive interference at specific angles. ŌĆó The electrons in an atom coherently scatter light. ŌĆō We can regard each atom as a coherent point scatterer ŌĆō The strength with which an atom scatters light is proportional to the number of electrons around the atom. ŌĆó The atoms in a crystal are arranged in a periodic array and thus can diffract light. ŌĆó The wavelength of X rays are similar to the distance between atoms. ŌĆó The scattering of X-rays from atoms produces a diffraction pattern, which contains information about the atomic arrangement within the crystal
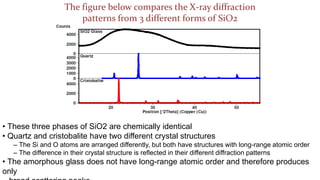
- 4. The figure below compares the X-ray diffraction patterns from 3 different forms of SiO2 ŌĆó These three phases of SiO2 are chemically identical ŌĆó Quartz and cristobalite have two different crystal structures ŌĆō The Si and O atoms are arranged differently, but both have structures with long-range atomic order ŌĆō The difference in their crystal structure is reflected in their different diffraction patterns ŌĆó The amorphous glass does not have long-range atomic order and therefore produces only
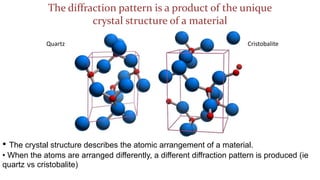
- 5. The diffraction pattern is a product of the unique crystal structure of a material Quartz Cristobalite ŌĆó The crystal structure describes the atomic arrangement of a material. ŌĆó When the atoms are arranged differently, a different diffraction pattern is produced (ie quartz vs cristobalite)
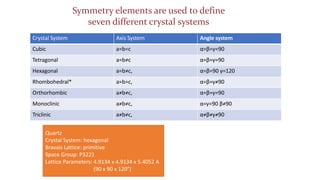
- 6. Crystalline materials are characterized by the long range orderly periodic arrangements of atoms. ŌĆó The unit cell is the basic repeating unit that defines the crystal structure. ŌĆō The unit cell contains the maximum symmetry that uniquely defines the crystal structure. ŌĆō The unit cell might contain more than one molecule: ŌĆó for example, the quartz unit cell contains 3 complete molecules of SiO2. ŌĆó The crystal system describes the shape of the unit cell ŌĆó The lattice parameters describe the size of the unit cell ŌĆó The unit cell repeats in all dimensions to fill space and produce the macroscopic grains or crystals of the material Crystal System: hexagonal Lattice Parameters: 4.9134 x 4.9134 x 5.4052 A (90 x 90 x 120┬░)
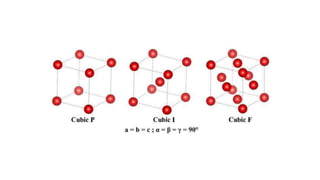
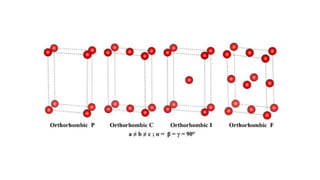
- 7. Crystal System Axis System Angle system Cubic a=b=c ╬▒=╬▓=╬│=90 Tetragonal a=bŌēĀc ╬▒=╬▓=╬│=90 Hexagonal a=bŌēĀc, ╬▒=╬▓=90 ╬│=120 Rhombohedral* a=b=c, ╬▒=╬▓=╬│ŌēĀ90 Orthorhombic aŌēĀbŌēĀc, ╬▒=╬▓=╬│=90 Monoclinic aŌēĀbŌēĀc, ╬▒=╬│=90 ╬▓ŌēĀ90 Triclinic aŌēĀbŌēĀc, ╬▒ŌēĀ╬▓ŌēĀ╬│ŌēĀ90 Quartz Crystal System: hexagonal Bravais Lattice: primitive Space Group: P3221 Lattice Parameters: 4.9134 x 4.9134 x 5.4052 A (90 x 90 x 120┬░) Symmetry elements are used to define seven different crystal systems
- 8. How are Crystal Systems Defined? There are six crystal systems. All minerals form crystals in one of these six systems. Although you may have seen more than six shapes of crystals, theyŌĆÖre all variations of one of these six habits. Each system is defined by a combination of three factors: ŌĆó How many axes it has. ŌĆó The lengths of the axes. ŌĆó The angles at which the axes meet. An axis is a direction between the sides. The shortest one is A. The longest is C. There is a B axis as well and sometimes a D axis.
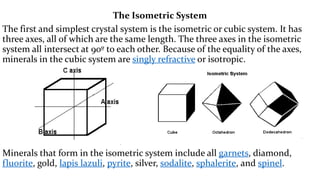
- 9. The Isometric System The first and simplest crystal system is the isometric or cubic system. It has three axes, all of which are the same length. The three axes in the isometric system all intersect at 90┬║ to each other. Because of the equality of the axes, minerals in the cubic system are singly refractive or isotropic. Minerals that form in the isometric system include all garnets, diamond, fluorite, gold, lapis lazuli, pyrite, silver, sodalite, sphalerite, and spinel.
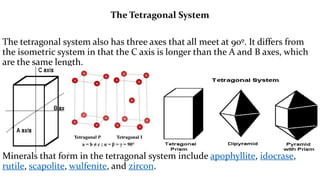
- 11. The Tetragonal System The tetragonal system also has three axes that all meet at 90┬║. It differs from the isometric system in that the C axis is longer than the A and B axes, which are the same length. Minerals that form in the tetragonal system include apophyllite, idocrase, rutile, scapolite, wulfenite, and zircon.
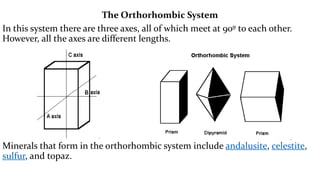
- 12. The Orthorhombic System In this system there are three axes, all of which meet at 90┬║ to each other. However, all the axes are different lengths. Minerals that form in the orthorhombic system include andalusite, celestite, sulfur, and topaz.
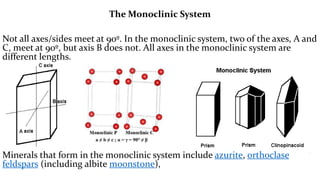
- 14. The Monoclinic System Not all axes/sides meet at 90┬║. In the monoclinic system, two of the axes, A and C, meet at 90┬║, but axis B does not. All axes in the monoclinic system are different lengths. Minerals that form in the monoclinic system include azurite, orthoclase feldspars (including albite moonstone),
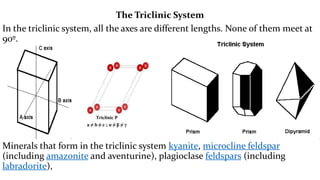
- 15. The Triclinic System In the triclinic system, all the axes are different lengths. None of them meet at 90┬║. Minerals that form in the triclinic system kyanite, microcline feldspar (including amazonite and aventurine), plagioclase feldspars (including labradorite),
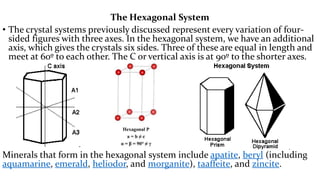
- 16. The Hexagonal System ŌĆó The crystal systems previously discussed represent every variation of four- sided figures with three axes. In the hexagonal system, we have an additional axis, which gives the crystals six sides. Three of these are equal in length and meet at 60┬║ to each other. The C or vertical axis is at 90┬║ to the shorter axes. Minerals that form in the hexagonal system include apatite, beryl (including aquamarine, emerald, heliodor, and morganite), taaffeite, and zincite.
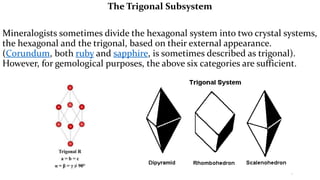
- 17. The Trigonal Subsystem Mineralogists sometimes divide the hexagonal system into two crystal systems, the hexagonal and the trigonal, based on their external appearance. (Corundum, both ruby and sapphire, is sometimes described as trigonal). However, for gemological purposes, the above six categories are sufficient.
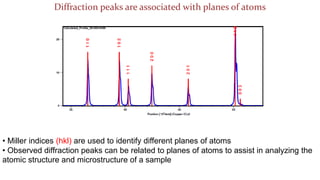
- 18. Diffraction peaks are associated with planes of atoms ŌĆó Miller indices (hkl) are used to identify different planes of atoms ŌĆó Observed diffraction peaks can be related to planes of atoms to assist in analyzing the atomic structure and microstructure of a sample
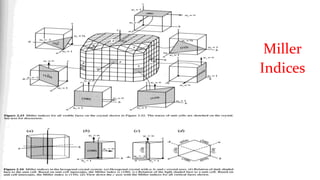
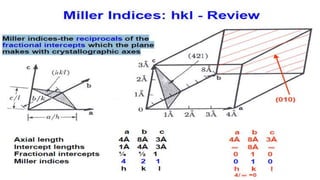
- 19. Miller Indices ŌĆó A workable symbolism results for the orientation of a plane in a lattice, the Miller indices, which are defined as the reciprocals of the fractional intercepts which the plane makes with the crystallographic axes. For example, if the Miller indices of a plane are (hkl), written in parentheses, then the plane makes fractional intercepts of l/h, l/k, l/l with the axes, and, if the axial lengths are a, b, c, the plane makes actual intercepts of a/h, b/k, c/l. ŌĆó Parallel to any plane in any lattice, there is a whole set of parallel equidistant planes, one of which passes through the origin; the Miller indices (hkl) usually refer to that plane in the set which is nearest the origin, although they may be taken as referring to any other plane in the set or to the whole set taken together.
- 20. Miller Indices
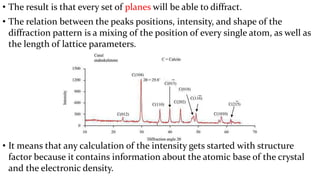
- 22. ŌĆó The result is that every set of planes will be able to diffract. ŌĆó The relation between the peaks positions, intensity, and shape of the diffraction pattern is a mixing of the position of every single atom, as well as the length of lattice parameters. ŌĆó It means that any calculation of the intensity gets started with structure factor because it contains information about the atomic base of the crystal and the electronic density.
- 23. Parallel planes of atoms intersecting the unit cell define directions and distances in the crystal. ŌĆó The Miller indices (hkl) define the reciprocal of the axial intercepts ŌĆó The crystallographic direction, [hkl], is the vector normal to (hkl) ŌĆó dhkl is the vector extending from the origin to the plane (hkl) and is normal to (hkl) ŌĆó The vector dhkl is used in BraggŌĆÖs law to determine where diffraction peaks will be The (200) planes of atoms in NaCl The (220) planes of atoms in NaCl
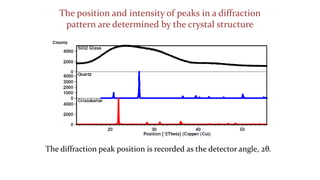
- 24. The position and intensity of peaks in a diffraction pattern are determined by the crystal structure The diffraction peak position is recorded as the detector angle, 2╬Ė.
- 25. The position of the diffraction peaks are determined by the distance between parallel planes of atoms. ŌĆó BraggŌĆÖs law calculates the angle where constructive interference from X-rays scattered by parallel planes of atoms will produce a diffraction peak. ŌĆō In most diffractometers, the X-ray wavelength l is fixed. ŌĆō Consequently, a family of planes produces a diffraction peak only at a specific angle 2q. ŌĆó dhkl is the vector drawn from the origin of the unit cell to intersect the crystallographic plane (hkl) at a 90┬░angle. ŌĆō dhkl, the vector magnitude, is the distance between parallel planes of atoms in the family (hkl) ŌĆō dhkl is a geometric function of the size and shape of the unit cell BraggŌĆÖs Law ╬╗= 2dhkl ØæĀØæ¢Øæø Ø£ā
- 26. A Brief Introduction to Miller Indices ŌĆó The Miller indices (hkl) define the reciprocal axial intercepts of a plane of atoms with the unit cell ŌĆó The (hkl) plane of atoms intercepts the unit cell at a/ŌäÄ, ØæÅ/Øæś, and ØæÉ/ØæÖ ŌĆó The (220) plane drawn to the right intercepts the unit cell at ┬Įa, ┬Įb, and does not intercept the c-axis. When a plane is parallel to an axis assumed to intercept at Ōł×; therefore its reciprocal is 0 ŌĆó The vector dhkl is drawn from the origin of the unit cell to intersect the crystallographic plane (hkl) at a 90┬░. ŌĆó The direction of dhkl is the crystallographic direction. ŌĆó The crystallographic direction is expressed using [] brackets, such as [220]
- 27. BraggŌĆÖs law provides a simplistic model to understand what conditions are required for diffraction. ŌĆó For parallel planes of atoms, with a space dhkl between the planes, constructive interference only occurs when BraggŌĆÖs law is satisfied. ŌĆō In our diffractometers, the X-ray wavelength l is fixed. ŌĆō Consequently, a family of planes produces a diffraction peak only at a specific angle 2q. ŌĆó Additionally, the plane normal [hkl] must be parallel to the diffraction vector s ŌĆō Plane normal [hkl]: the direction perpendicular to a plane of atoms ŌĆō Diffraction vector s: the vector that bisects the angle between the incident and diffracted beam BraggŌĆÖs Law ╬╗= 2dhkl ØæĀØæ¢Øæø Ø£ā
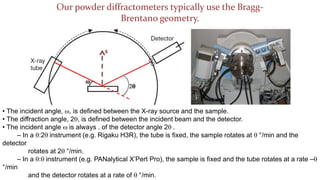
- 28. Our powder diffractometers typically use the Bragg- Brentano geometry. ŌĆó The incident angle, w, is defined between the X-ray source and the sample. ŌĆó The diffraction angle, 2q, is defined between the incident beam and the detector. ŌĆó The incident angle w is always . of the detector angle 2q . ŌĆō In a q:2q instrument (e.g. Rigaku H3R), the tube is fixed, the sample rotates at q ┬░/min and the detector rotates at 2q ┬░/min. ŌĆō In a q:q instrument (e.g. PANalytical XŌĆÖPert Pro), the sample is fixed and the tube rotates at a rate ŌĆōq ┬░/min and the detector rotates at a rate of q ┬░/min.
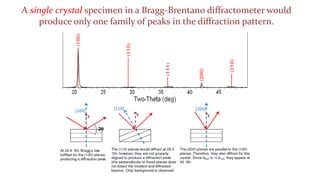
- 29. A single crystal specimen in a Bragg-Brentano diffractometer would produce only one family of peaks in the diffraction pattern.
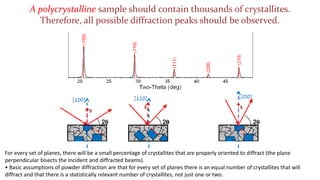
- 30. For every set of planes, there will be a small percentage of crystallites that are properly oriented to diffract (the plane perpendicular bisects the incident and diffracted beams). ŌĆó Basic assumptions of powder diffraction are that for every set of planes there is an equal number of crystallites that will diffract and that there is a statistically relevant number of crystallites, not just one or two. A polycrystalline sample should contain thousands of crystallites. Therefore, all possible diffraction peaks should be observed.
- 31. The spacing of one set of crystal planes in NaCl (table salt) is d = 0.282 nm. A monochromatic beam of X-rays produces a Bragg maximum when its glancing angle with these planes is = 7. Assuming that this is a first order maximum (n = 1), find the wavelength of the X-rays. The Bragg law is 2d sin = n╬╗ ╬╗ = 2d sin = 2 ├Ś (0.282 nm) ├Ś sin 7 ╬╗ = 0.069 nm
- 32. 1. A beam of X-rays of wavelength 0.071 nm is diffracted by (110) plane of rock salt with lattice constant of 0.28 nm. Find the glancing angle for the second-order diffraction. Sol: Given data are: Wavelength (╬╗) of X-rays = 0.071 nm Lattice constant (a) = 0.28 nm Plane (hkl) = (110) Order of diffraction = 2 Glancing angle ╬Ė = ? BraggŌĆÖs law is 2d sin ╬Ė = n╬╗






![Parallel planes of atoms intersecting the unit cell define
directions and distances in the crystal.
ŌĆó The Miller indices (hkl) define the reciprocal of the axial intercepts
ŌĆó The crystallographic direction, [hkl], is the vector normal to (hkl)
ŌĆó dhkl is the vector extending from the origin to the plane (hkl) and is normal to (hkl)
ŌĆó The vector dhkl is used in BraggŌĆÖs law to determine where diffraction peaks will be
The (200) planes
of atoms in NaCl
The (220) planes
of atoms in NaCl](https://image.slidesharecdn.com/introductiontocrystallographyandx-raydiffractiontheory1-200219093112/85/Introduction-to-crystallography-and-x-ray-diffraction-theory-23-320.jpg)

![A Brief Introduction to Miller Indices
ŌĆó The Miller indices (hkl) define the reciprocal axial
intercepts of a plane of atoms with the unit cell
ŌĆó The (hkl) plane of atoms intercepts the unit cell at a/ŌäÄ,
ØæÅ/Øæś, and ØæÉ/ØæÖ
ŌĆó The (220) plane drawn to the right intercepts the unit
cell at ┬Įa, ┬Įb, and does not intercept the c-axis. When a plane is parallel to
an axis assumed to intercept at Ōł×; therefore its
reciprocal is 0
ŌĆó The vector dhkl is drawn from the origin of the unit cell
to intersect the crystallographic plane (hkl) at a 90┬░.
ŌĆó The direction of dhkl is the crystallographic direction.
ŌĆó The crystallographic direction is expressed using [] brackets, such as [220]](https://image.slidesharecdn.com/introductiontocrystallographyandx-raydiffractiontheory1-200219093112/85/Introduction-to-crystallography-and-x-ray-diffraction-theory-26-320.jpg)
![BraggŌĆÖs law provides a simplistic model to understand
what conditions are required for diffraction.
ŌĆó For parallel planes of atoms, with a space dhkl between the planes, constructive
interference only occurs when BraggŌĆÖs law is satisfied.
ŌĆō In our diffractometers, the X-ray wavelength l is fixed.
ŌĆō Consequently, a family of planes produces a diffraction peak only at a specific angle 2q.
ŌĆó Additionally, the plane normal [hkl] must be parallel to the diffraction vector s
ŌĆō Plane normal [hkl]: the direction perpendicular to a plane of atoms
ŌĆō Diffraction vector s: the vector that bisects the angle between the incident and diffracted beam
BraggŌĆÖs Law
╬╗= 2dhkl ØæĀØæ¢Øæø Ø£ā](https://image.slidesharecdn.com/introductiontocrystallographyandx-raydiffractiontheory1-200219093112/85/Introduction-to-crystallography-and-x-ray-diffraction-theory-27-320.jpg)