JJ Thomson: The Plum Pudding Model
- 1. JJ Thomson: the PLUM PUDDING MODEL!!! Michelle Kao Elysia Hung
- 2. Plum pudding is not too bad to eat JJ ThomsonŌĆÖs Plum Pudding Model is not too bad to see
- 3. Index!! =D J.J. Thomson Cathode Ray Tube (CRT) Cathode Rays!! And theŌĆ”.amazingŌĆ” Plum Pudding Model!! \@/ AndŌĆ”theŌĆ” DaltonŌĆÖs ModelŌĆ” ’üī
- 4. J.J. Thomson is a British Physicist and a Nobel Prize Winner who discovered electrons and isotopes in 1897. And Died in 1940. ’üī Background Information
- 5. <-- J.J. ThomsonŌĆÖs true formŌĆ” just kidding~
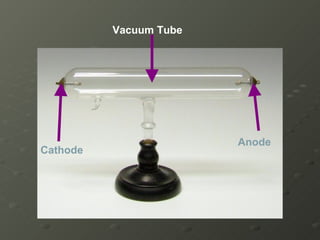
- 6. He used a Cathode Ray Tube (CRT)
- 7. Vacuum Tube Cathode Anode
- 9. Then, what is a cathode ray?? The glow is actually many many many many many many many many many many many many many many many many many many many many many many many many many many many many many many ELECTRONS!
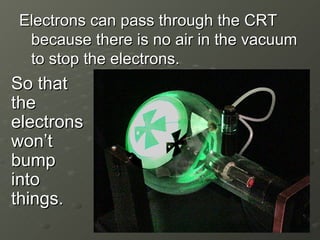
- 10. Electrons can pass through the CRT because there is no air in the vacuum to stop the electrons. So that the electrons wonŌĆÖt bump into things.
- 11. SO BEAUTIFUL~
- 12. This is one puddingŌĆÖs sad, short storyŌĆ” Intermission~ A story about a freedom longed for, but never attainedŌĆ”
- 13. ╠²
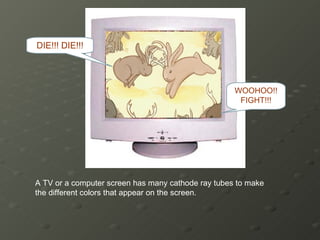
- 14. A TV or a computer screen has many cathode ray tubes to make the different colors that appear on the screen. WOOHOO!! FIGHT!!! DIE!!! DIE!!!
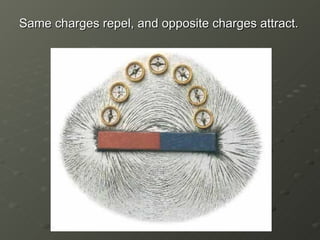
- 15. Same charges repel, and opposite charges attract.
- 16. Magnet The ray arcs away from the magnet
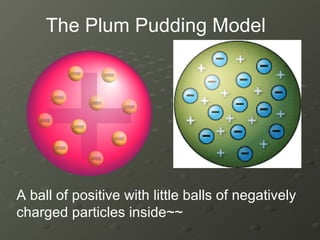
- 17. A ball of positive with little balls of negatively charged particles inside~~ The Plum Pudding Model
- 18. ╠²
- 19. ╠²
- 20. DaltonŌĆÖs Model Dalton believes that an atom is a solid ball. Each elements are different shaped balls since each atom has a different property.
- 21. Thomson switch the cathode and anode with different metals and found out that they all create a cathode ray. He concluded that whatever is present in an atom is also in all the other atoms. (electrons~)
- 22. SUMMARY TIME!! YAHOO! J.J. Thomson discovered electrons in 1897. Cathode Ray Tubes are vacuums with cathode and anode. The ray arcs away when a ŌĆō magnet is close by. Plum pudding model is a ball with electrons stuck in it. He disproved DaltonŌĆÖs Model.
- 23. Farewell! Thanks for watching! Bye bye!