kinetics-lecture3.pdf
0 likes51 views
The document discusses determining reaction orders from rate laws and experimental data. It provides examples of using rate law data to calculate the individual reaction orders with respect to each reactant and the overall order. The key points are: - Reaction orders are determined by the exponents in the rate law, not the coefficients in the balanced chemical equation. - To determine the order with respect to a reactant, experiments are run where its concentration is varied while others are held constant. - Orders can be found by taking the ratio of rates and solving equations relating rate changes to concentration changes. - Reaction orders can be positive integers, zero, negative integers, or fractions.
1 of 16
Download to read offline
![Individual and Overall Reaction Orders
For the reaction 2NO(g) + 2H2(g) ŌåÆ N2(g) + 2H2O(g):
The rate law is rate = k[NO]2[H2]
The reaction is second order with respect to NO, first
order with respect to H2 and third order overall.
Note that the reaction is first order with respect to H2
even though the coefficient for H2 in the balanced
equation is 2.
Reaction orders must be determined from experimental
data and cannot be deduced from the balanced
equation. Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-1-320.jpg)
![PLAN: We inspect the exponents in the rate law, not the coefficients
of the balanced equation, to find the individual orders. We add
the individual orders to get the overall reaction order.
SOLUTION:
(a) The exponent of [NO] is 2 and the exponent of [O2] is 1, so the
reaction is second order with respect to NO, first order
with respect to O2 and third order overall.
Determining Reaction Orders from Rate Laws
PROBLEM2: For each of the following reactions, use the give rate law
to determine the reaction order with respect to each
reactant and the overall order.
(a) 2NO(g) + O2(g) ŌåÆ 2NO2(g); rate = k[NO]2[O2]
(b) CH3CHO(g) ŌåÆ CH4(g) + CO(g); rate = k[CH3CHO]3/2
(c) 3
H2O2(aq) + 3I-(aq) + 2H+(aq) ŌåÆI -(aq) + 2H2O(l); rate = k[H2O2][I-]
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-2-320.jpg)

![Determining Reaction Orders
For the general reaction A + 2B ŌåÆ C +D,
the rate law will have the form
Rate = k[A]m[B]n
Todetermine the values of m and n, we run a series of
experiments in which one reactant concentration
changes while the other is kept constant, and we
measure the effect on the initial rate in each case.
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-4-320.jpg)
![Table 2 Initial Rates for the Reaction between A and B
Initial Rate Initial [A] Initial [B]
Experiment (mol/L┬Ęs) (mol/L) (mol/L)
1 1.75x10-3 2.50x10-2 3.00x10-2
2 3.50x10-3 5.00x10-2 3.00x10-2
3 3.50x10-3 2.50x10-2 6.00x10-2
4 7.00x10-3 5.00x10-2 6.00x10-2
[B] is kept constant for experiments 1 and 2, while [A] is doubled.
Then [A] is kept constant while [B] is doubled.
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-5-320.jpg)
![Rate 2
Rate 1
=
k[A] m [B]n
2 2
1
k[A] m
[B]n
1
Finding m, the order with respect to A:
We compare experiments 1 and 2, where [B] is kept
constant but [A] doubles:
=
[A]
m
2
m
[A]1
=
[A]2
m
3.50x10-3 mol/L┬Ęs
1.75x10-3mol/L┬Ęs
=
5.00x10-2 mol/L
2.50x10-2 mol/L
[A]1
m
Dividing, we get 2.00 = (2.00)m so m = 1
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-6-320.jpg)
![Rate 3
Rate 1
=
k[A] m [B]n
3 3
1
k[A] m
[B]n
1
Finding n, the order with respect to B:
We compare experiments 3 and 1, where [A] is kept
constant but [B] doubles:
=
[B]
n
3
n
[B]1
=
[B]3
n
3.50x10-3 mol/L┬Ęs
1.75x10-3mol/L┬Ęs
=
6.00x10-2 mol/L
3.00x10-2 mol/L
[B]1
m
Dividing, we get 2.00 = (2.00)n so n = 1
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-7-320.jpg)
![Table 3 Initial Rates for the Reaction between O2 and NO
O2(g) + 2NO(g) ŌåÆ 2NO2(g) Rate = k[O2]m[NO]n
Initial Rate
Initial Reactant
Concentrations (mol/L)
Experiment (mol/L┬Ęs) [O2] [NO]
1 3.21x10-3 1.10x10-2 1.30x10-2
2 6.40x10-3 2.20x10-2 1.30x10-2
3 12.48x10-3 1.10x10-2 2.60x10-2
4 9.60x10-3 3.30x10-2 1.30x10-2
5 28.8x10-3 1.10x10-2 3.90x10-2
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-8-320.jpg)
![Rate 2
Rate 1
= 2 2
2 1
k[O ] m
[NO]n
1
Finding m, the order with respect to O2:
We compare experiments 1 and 2, where [NO] is kept
constant but [O2] doubles:
=
k[O2] m [NO]n [O2] m
[O ]m
2 1
2 =
[O ]
2 2
[O ]
2 1
m
6.40x10-3 mol/L┬Ęs
=
2.20x10-2 mol/L
3.21x10-3mol/L┬Ęs 1.10x10-2 mol/L
Dividing, we get 1.99 = (2.00)m or 2 = 2m, so m = 1
The reaction is first order with respect to O2.
m
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-9-320.jpg)

![Finding n, the order with respect to NO:
We compare experiments 1 and 3, where [O2] is kept
constant but [NO] doubles:
Rate 3
=
[NO]3
Rate 1 [NO]1
n
=
12.8x10-3 mol/L┬Ęs 2.60x10-2 mol/L
3.21x10-3mol/L┬Ęs 1.30x10-2 mol/L
n
Dividing, we get 3.99 = (2.00)n or 4 = 2n, so n =2.
The reaction is second order with respect to NO.
The rate law is given by: rate = k[O2][NO]2
log a
=
log 3.99
log b log 2.00
n = = 2.00
Alternatively:
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-11-320.jpg)
![Sample Problem 3 Determining Reaction Orders from Rate Data
PROBLEM: Many gaseous reactions occur in a car engine and exhaust
system. One of these reactions is
NO2(g) + CO(g) ŌåÆ NO(g) + CO2(g) rate = k[NO2]m[CO]n
Use the following data to determine the individual and
overall reaction orders:
Experiment
Initial Rate
(mol/L┬Ęs)
Initial [NO2]
(mol/L)
Initial [CO]
(mol/L)
1 0.0050 0.10 0.10
2 0.080 0.40 0.10
3 0.0050 0.10 0.20
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-12-320.jpg)
![Sample Problem 3
PLAN: We need to solve the general rate law for m and for n and
then add those orders to get the overall order. We proceed by
taking the ratio of the rate laws for two experiments in which
only the reactant in question changes concentration.
rate 2
rate 1 [NO ]
2 1
k [NO ]m [CO]n [NO ]
2 2 2
= 2 2
1
k [NO2]m [CO]n
1
=
0.080 0.40
0.0050 0.10
=
m
SOLUTION:
Tocalculate m, the order with respect to NO2, we compare
experiments 1 and 2:
m
16 = (4.0)m so m = 2
The reaction is second order in NO2.
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-13-320.jpg)
![k [NO ]m [CO]n [CO] 3
[CO]1
2 3 3
=
k [NO2]m [CO]n
1 1
n
rate 3
rate 1
= =
0.0050 0.20
0.0050 0.10
n
1.0 = (2.0)n so n = 0
The reaction is zero order in CO.
rate = k[NO2]2[CO]0 or rate = k[NO2]2
Sample Problem 3
Tocalculate n, the order with respect to CO, we compare experiments
1 and 3:
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-14-320.jpg)
![Sample Problem 4
SOLUTION:
(a) For reactant A(red):
Experiments 1 and 2 have the same number of particles of B, but
the number of particles of A doubles. The rate doubles. Thus the
order with respect to A is 1.
For reactant B (blue):
Experiments 1 and 3 show that when the number of particles of B
doubles (while A remains constant), the rate quadruples. The
order with respect to B is 2.
The overall order is 1 + 2 = 3.
(b) Rate = k[A][B]2
(c) Between experiments 3 and 4, the number of particles of A
doubles while the number of particles of B does not change. The
rate should double, so rate = 2 x 2.0x10-4 = 4.0x10-4mol/L┬Ęs
Dr. N. Singh](https://image.slidesharecdn.com/kinetics-lecture3-220901050303-f2332f4a/85/kinetics-lecture3-pdf-16-320.jpg)
Ad
Recommended
Chemical kinetics_Rate laws and reaction mechanisms.pdf
Chemical kinetics_Rate laws and reaction mechanisms.pdfChimwemweGladysBanda
╠²
La cin├®tica qu├Łmica estudia la velocidad de las reacciones qu├Łmicas, centr├Īndose en la tasa de consumo de reactivos y formaci├│n de productos, as├Ł como la influencia de condiciones de reacci├│n. Se define la tasa de reacci├│n como el cambio en la concentraci├│n de reactivos o productos por unidad de tiempo, y se utiliza la ley de la velocidad para relacionar estas tasas con las concentraciones de reactivos. Se determina el orden de reacci├│n a partir de datos experimentales y puede aceptarse un orden fraccional en algunos casos.Lect w2 152 - rate laws_alg
Lect w2 152 - rate laws_algchelss
╠²
This document discusses reaction rates and kinetics concepts including:
- Instantaneous reaction rates can be calculated from the slope of concentration-time graphs at specific points.
- Reaction orders and rate laws can be determined experimentally using methods like the initial rate method or integrated rate law method.
- First-order reactions follow the integrated rate law that the natural log of the concentration is linear with time. Second-order and zero-order reactions also have defining rate laws and kinetics equations.Ch14-kinetics chemistry chemical lecture.pdf
Ch14-kinetics chemistry chemical lecture.pdfRozinaSana1
╠²
This chapter discusses chemical kinetics and reaction rates. It introduces concepts such as:
- Reaction rate is measured by how concentrations change over time.
- Factors that influence reaction rate include concentration, physical state, temperature, and surface area.
- The rate law expresses reaction rate in terms of reactant concentrations and a rate constant. It has the form: rate = k[A]m[B]n, where m and n are the reaction orders.
- Reaction orders can be determined experimentally by varying one reactant concentration at a time and observing how the rate changes.8.1 rate law
8.1 rate lawsathiakumaran
╠²
The document discusses reaction kinetics and rate laws. It defines key terms like rate law, order of reaction, and rate constant. The rate law expresses the relationship between the rate of a reaction and the concentrations of reactants raised to powers corresponding to their order. The order of a reaction with respect to a reactant is the exponent on its concentration term in the rate expression. The total order is the sum of all exponents. Examples are provided to demonstrate how to determine orders from rate laws and write rate expressions.chapt16_lecture.ppt
chapt16_lecture.pptAbrahamMoraTumanggor
╠²
This document discusses key concepts in chemical kinetics including:
- Reaction rates can be expressed as changes in reactant or product concentrations over time.
- Factors that influence reaction rates include concentration, physical state, and temperature. Higher concentrations, smaller particle sizes, and higher temperatures typically increase reaction rates.
- The rate law expresses reaction rate as a function of reactant concentrations and can reveal information about the reaction mechanism. It is determined experimentally by measuring initial rates under different conditions.Chemical Kinetics for students 1-2 years
Chemical Kinetics for students 1-2 yearsssusere91cce
╠²
The document discusses chemical kinetics, focusing on the rates and mechanisms of chemical reactions, and how to experimentally determine rate laws using initial concentrations and reaction rates. It explains the significance of rate laws, the calculation of rate constants, and the difference between first-order and second-order reactions. Additionally, the document describes the reaction mechanisms and the steady-state approximation in simplifying complex reaction systems.Initial rate method
Initial rate methodsathiakumaran
╠²
The document discusses determining the order of reaction using the initial rate method. It provides examples of experimental data collected for reactions, including initial concentrations of reactants and measured initial rates. It then shows solutions for determining the order of reactions and rate constants by analyzing changes in initial rates with changing concentrations. Key steps include writing rate laws, comparing reaction rates between experiments, and calculating exponents from rate laws. The overall goal is to demonstrate how to apply the initial rate method to experimental data to determine reaction orders and rate constants.Chapter_12_Chemical_Kinetics.ppt
Chapter_12_Chemical_Kinetics.pptErnest Obese
╠²
This document discusses key concepts in chemical kinetics including:
- Rate laws describe how reaction rates depend on reactant concentrations. Rate laws can be determined experimentally from initial rate data.
- Reactions can be zero-order, first-order, or second-order depending on how the rate depends on reactant concentrations.
- Integrated rate laws relate reactant concentrations to time and rate constants. Graphical methods using plots of concentration or transformed concentration vs. time can determine reaction order and rate constants.Chapter 5_Chemical Kinetics_ GENERAL_ CHEMISTRY
Chapter 5_Chemical Kinetics_ GENERAL_ CHEMISTRYMinhQuan85
╠²
Chapter 5 of the document focuses on kinetic chemistry, explaining the distinction between thermodynamics and kinetics. It details factors influencing reaction rates, such as reactant concentration, temperature, and catalysts, and presents various methods for determining reaction rates and orders. The chapter concludes with the effects of temperature and concentration on reaction rates, including the interpretation of the Arrhenius equation.c5-chemkinetic_ko_thi_effect_of_temperature_and_concentration.pptx
c5-chemkinetic_ko_thi_effect_of_temperature_and_concentration.pptxkhoinguyenngoccs3008
╠²
1) The document discusses kinetic chemistry, including reaction rates, rate constants, reaction orders, and factors that affect reaction rates such as concentration, temperature, surface area, and catalysts.
2) It provides examples of determining reaction orders based on changes in reaction rate with changes in concentration. Reaction orders can be fractional, zero, or higher than one.
3) The rate constant k depends on temperature according to the Arrhenius equation, and increases with higher temperature based on the activation energy of the reaction.Chemistry note : Chemical Kinetics grade 11
Chemistry note : Chemical Kinetics grade 11elia7namweya
╠²
The document discusses chemical kinetics, focusing on reaction rates, the relationship between concentration and reaction speed, and how to determine rate laws and reaction orders experimentally. It explains the importance of kinetics in fully describing chemical reactions, including the effects of temperature, catalysts, and concentration on reaction rates. Various examples and formulations illustrate how to analyze and measure reaction rates and the significance of rate laws in understanding chemical processes.Ch12 z5e kinetics
Ch12 z5e kineticsblachman
╠²
This document discusses reaction kinetics and rate laws. It explains that the rate of a reaction can be defined based on the disappearance of reactants or appearance of products over time. The rate law expression relates the reaction rate to the concentrations of reactants and has the form Rate = k[A]n[B]m, where k is the rate constant and n and m are the orders of reactants A and B. The orders must be determined experimentally by measuring initial rates as concentrations are varied. A first-order reaction has a rate that depends on just one reactant concentration. Integrating the differential rate law gives an integrated rate law relating concentration to time, such as the first-order rate law ln[A] = -kt + lnch16_lecture_8e.pptx
ch16_lecture_8e.pptxKETEM1
╠²
This document provides an overview of chemical kinetics and reaction rates from the textbook "Chemistry: The Molecular Nature of Matter and Change". It discusses key topics such as:
- Factors that influence reaction rates like concentration, temperature, and surface area
- Expressing reaction rates in terms of changes in concentration over time
- Determining the rate law and reaction orders from experimental data
- Differentiating between zero, first, and second-order reactions based on their rate equations
- Calculating changes in reaction rates when concentrations are varied
The document uses diagrams, examples, and sample problems to illustrate concepts in chemical kinetics and determining reaction mechanisms and orders from rate data.Chemical kinetics
Chemical kineticsRajveer Bhaskar
╠²
This document provides background information on reaction rates and mechanisms. It discusses how factors like reactant concentrations, temperature, catalysts, and surface area can influence reaction rates. It also defines concepts like the rate law, rate constant, reaction order, energy of activation, and Arrhenius equation. Methods for determining reaction order are described, including by varying reactant concentrations and analyzing integrated rate expressions for zero, first, and second order reactions. The effects of temperature on reaction rates are also addressed through the Arrhenius equation.Chemical kinetics and reaction lecture notes
Chemical kinetics and reaction lecture notesMaameDurowaa
╠²
The document covers key concepts in chemical kinetics, including the expression of reaction rates, determination of reaction orders, and the methods used to derive rate laws. It outlines various types of rates (initial, instantaneous, and average) and emphasizes the importance of rate laws in understanding reaction mechanisms at a molecular level. Additionally, it details the graphical methods for determining rate laws and explores the half-lives of reactions and the relationship between concentration and reaction rates.Chemical Kinetics
Chemical Kineticsjc762006
╠²
This document summarizes key concepts in chemical kinetics including:
1) Kinetics is the study of reaction rates and how the molecular mechanism influences the rate. Factors like temperature, concentration, and catalysts affect the reaction rate.
2) The rate of a reaction is defined as the change in concentration of a reactant or product over time. Rate laws relate the reaction rate to concentrations of reactants.
3) Integrated rate laws allow calculation of reactant/product concentration as a function of time for different reaction orders (zero, first, second order). Graphical methods using these relations can determine the reaction order and rate constant.chemical-kinetics-ppt
chemical-kinetics-pptDaizyDmello2
╠²
This document provides an overview of chemical kinetics, including:
- Reaction rates are determined by how concentrations of reactants and products change over time.
- The rate law expresses the relationship between reaction rate and concentrations of reactants. Rate laws are determined experimentally.
- Reactions can be zero-order, first-order, or second-order depending on how the rate depends on concentrations.
- Reaction mechanisms involve elementary steps that describe reactions at the molecular level.
- Catalysts increase reaction rates by lowering the activation energy without being consumed in the process.Rate of chemical reaction
Rate of chemical reactionRAJEEVBAYAN1
╠²
Here are the steps to determine the order of the reaction:
1) Plot [X] vs time on a graph. You will get a straight line through the origin, indicating the reaction is first order.
2) Take the log of both sides of the rate law equation:
Rate = k[X]
Log(Rate) = Log(k[X])
3) Plot log(Rate) vs log([X]). You will get a straight line with a slope of 1, confirming the reaction is first order.
Therefore, based on the experimental data and analysis, this reaction is first order with respect to X.Kinetics ppt
Kinetics pptekozoriz
╠²
This document provides an overview of chemical kinetics and reaction rates. It discusses topics such as reaction rate, rate laws, reaction orders, rate constants, factors that affect reaction rates like temperature, catalysts, and enzyme kinetics. Specific examples are also provided to illustrate concepts like first-order and second-order reactions, reaction mechanisms, and industrial catalytic processes like the Haber process and catalytic converters.Kinetics.pptx
Kinetics.pptxFatmaMoustafa6
╠²
Chemical kinetics is the study of reaction rates and mechanisms. Key aspects include determining how factors like temperature, pressure, catalysts and light influence reaction rates. Reaction rates are determined by monitoring changes in reactant or product concentration over time. The rate of a reaction depends on the concentrations of reactants and can be modeled using a rate law. Common reaction orders include zero-order, first-order and second-order reactions, which have different relationships between rate and concentration. Catalysts increase reaction rates by providing an alternative reaction pathway with a lower activation energy.AP Chemistry Chapter 14 Powerpoint for website.ppt
AP Chemistry Chapter 14 Powerpoint for website.pptKimberlyAnnePagdanga1
╠²
This document summarizes key concepts in chemical kinetics. It discusses factors that affect reaction rates such as concentration, temperature, and catalysts. Reaction rates are expressed using rate laws and rate constants. Rate laws are determined experimentally and show how reaction rates depend on concentrations. Rates change over time and can be modeled using integrated rate laws for first and second order reactions. Reaction mechanisms involve elementary steps and may have intermediates. The activation energy required to reach the transition state affects temperature dependence of reaction rates.Chapter 17.4 : Reaction Rate
Chapter 17.4 : Reaction RateChris Foltz
╠²
This document provides information about chemical kinetics and reaction rates. It defines chemical kinetics and discusses the two conditions needed for reactions to occur: contact between particles and enough energy for activation. Reaction rate depends on collision frequency and efficiency of reactants and is influenced by nature of reactants, surface area, temperature, concentration, and presence of a catalyst. It also defines catalysts and different types. The relationship between reaction rate and concentration is given by the rate law, and order of a reaction relates to its rate law. Rate laws are written for different reaction examples and mechanisms.Chemical kinetics
Chemical kineticsDrgholamabbasChehard
╠²
1) This document summarizes key concepts from a lecture on chemical kinetics including reaction rates, rate laws, reaction mechanisms, and a collision model for chemical reactions.
2) Reaction rates describe how fast concentrations of reactants and products change over time. Rate laws define the relationship between reaction rate and concentrations of reactants.
3) Reaction mechanisms involve elementary steps that may involve intermediates to ultimately form products. The rate-determining step controls the overall rate.Chapter 12 chemical kinetics2
Chapter 12 chemical kinetics2DrgholamabbasChehard
╠²
1) This document summarizes key concepts from a lecture on chemical kinetics including reaction rates, rate laws, reaction mechanisms, and a collision model for chemical reactions.
2) Reaction rates describe how fast concentrations of reactants and products change over time. Rate laws define the relationship between reaction rate and concentrations of reactants.
3) Reaction mechanisms involve elementary steps that may involve intermediates to ultimately form products. The rate-determining step controls the overall rate.Chemical Kinetics chemical kinetics chemistry.ppt
Chemical Kinetics chemical kinetics chemistry.pptFatmaMoustafa6
╠²
Chemical Kinetics chemical kinetics chemistry.pptOrder of reaction and rate of reaction
Order of reaction and rate of reactionAhmad Hassan
╠²
The document discusses reaction order and methods for determining reaction order. It defines reaction order as the power dependence of the rate of reaction on the concentrations of reactants. Reaction order can be determined using the initial rates method, integral method, or differential method. Different reaction orders are also described, including zero-order reactions, where concentration does not affect rate; first-order reactions, where rate depends on one reactant; and second-order reactions, where rate depends on two reactants or one reactant squared. Pseudo-first order reactions are also discussed.Abc Kinetics
Abc Kineticsdluetgens
╠²
The document discusses kinetics and reaction rates. It defines kinetics as the study of reaction rates, and explains that thermodynamics determines if a reaction will occur but not how fast. It then covers factors that affect reaction rates like temperature, concentration, and catalysts. The document provides examples of calculating rates from concentration data and determining rate laws from experimental results. It also introduces integrated rate laws and how to determine reaction order from different rate law equations.Chemistry Lec-18A-19AB.pptx
Chemistry Lec-18A-19AB.pptxabdulahad563527
╠²
The document discusses reaction rates and orders. It states that the rate law can be used to calculate reaction rates if the rate constant (k), reaction order (x and y), and reactant concentrations are known. The overall reaction order is the sum of the powers of the reactant concentrations in the rate law. Reaction orders must be determined experimentally and cannot be deduced from the balanced reaction equation alone. Graphical methods can be used to determine the reaction order based on the shape of plots of concentration vs. time.kinetics-lecture6.pdf
kinetics-lecture6.pdfDr Nirankar Singh
╠²
This document discusses key concepts relating to reaction rates:
- The half-life of a first-order reaction does not depend on initial concentration, while half-lives of second and zero-order reactions do depend on initial concentration.
- Reaction rates increase with higher concentrations because there are more collisions between reactant particles.
- Temperature also affects reaction rates because it increases the kinetic energy of particles, resulting in more frequent collisions exceeding the activation energy.
- The Arrhenius equation quantifies the exponential relationship between temperature and the rate constant.kinetics-lecture5.pdf
kinetics-lecture5.pdfDr Nirankar Singh
╠²
The document discusses reaction order and integrated rate laws for chemical reactions. It provides examples of determining the reaction order from concentration-time data and calculating rate constants from half-life. Specifically:
- The reaction order for N2O5 decomposition is determined to be first-order from a plot of ln[N2O5] vs. time.
- Integrated rate laws are presented for zero-order, first-order, and second-order reactions relating the change in concentration of a reactant over time.
- Half-life is defined as the time for a reactant concentration to reduce to half its initial value, and the equation to calculate half-life from the rate constant is given for firstMore Related Content
Similar to kinetics-lecture3.pdf (20)
Chapter 5_Chemical Kinetics_ GENERAL_ CHEMISTRY
Chapter 5_Chemical Kinetics_ GENERAL_ CHEMISTRYMinhQuan85
╠²
Chapter 5 of the document focuses on kinetic chemistry, explaining the distinction between thermodynamics and kinetics. It details factors influencing reaction rates, such as reactant concentration, temperature, and catalysts, and presents various methods for determining reaction rates and orders. The chapter concludes with the effects of temperature and concentration on reaction rates, including the interpretation of the Arrhenius equation.c5-chemkinetic_ko_thi_effect_of_temperature_and_concentration.pptx
c5-chemkinetic_ko_thi_effect_of_temperature_and_concentration.pptxkhoinguyenngoccs3008
╠²
1) The document discusses kinetic chemistry, including reaction rates, rate constants, reaction orders, and factors that affect reaction rates such as concentration, temperature, surface area, and catalysts.
2) It provides examples of determining reaction orders based on changes in reaction rate with changes in concentration. Reaction orders can be fractional, zero, or higher than one.
3) The rate constant k depends on temperature according to the Arrhenius equation, and increases with higher temperature based on the activation energy of the reaction.Chemistry note : Chemical Kinetics grade 11
Chemistry note : Chemical Kinetics grade 11elia7namweya
╠²
The document discusses chemical kinetics, focusing on reaction rates, the relationship between concentration and reaction speed, and how to determine rate laws and reaction orders experimentally. It explains the importance of kinetics in fully describing chemical reactions, including the effects of temperature, catalysts, and concentration on reaction rates. Various examples and formulations illustrate how to analyze and measure reaction rates and the significance of rate laws in understanding chemical processes.Ch12 z5e kinetics
Ch12 z5e kineticsblachman
╠²
This document discusses reaction kinetics and rate laws. It explains that the rate of a reaction can be defined based on the disappearance of reactants or appearance of products over time. The rate law expression relates the reaction rate to the concentrations of reactants and has the form Rate = k[A]n[B]m, where k is the rate constant and n and m are the orders of reactants A and B. The orders must be determined experimentally by measuring initial rates as concentrations are varied. A first-order reaction has a rate that depends on just one reactant concentration. Integrating the differential rate law gives an integrated rate law relating concentration to time, such as the first-order rate law ln[A] = -kt + lnch16_lecture_8e.pptx
ch16_lecture_8e.pptxKETEM1
╠²
This document provides an overview of chemical kinetics and reaction rates from the textbook "Chemistry: The Molecular Nature of Matter and Change". It discusses key topics such as:
- Factors that influence reaction rates like concentration, temperature, and surface area
- Expressing reaction rates in terms of changes in concentration over time
- Determining the rate law and reaction orders from experimental data
- Differentiating between zero, first, and second-order reactions based on their rate equations
- Calculating changes in reaction rates when concentrations are varied
The document uses diagrams, examples, and sample problems to illustrate concepts in chemical kinetics and determining reaction mechanisms and orders from rate data.Chemical kinetics
Chemical kineticsRajveer Bhaskar
╠²
This document provides background information on reaction rates and mechanisms. It discusses how factors like reactant concentrations, temperature, catalysts, and surface area can influence reaction rates. It also defines concepts like the rate law, rate constant, reaction order, energy of activation, and Arrhenius equation. Methods for determining reaction order are described, including by varying reactant concentrations and analyzing integrated rate expressions for zero, first, and second order reactions. The effects of temperature on reaction rates are also addressed through the Arrhenius equation.Chemical kinetics and reaction lecture notes
Chemical kinetics and reaction lecture notesMaameDurowaa
╠²
The document covers key concepts in chemical kinetics, including the expression of reaction rates, determination of reaction orders, and the methods used to derive rate laws. It outlines various types of rates (initial, instantaneous, and average) and emphasizes the importance of rate laws in understanding reaction mechanisms at a molecular level. Additionally, it details the graphical methods for determining rate laws and explores the half-lives of reactions and the relationship between concentration and reaction rates.Chemical Kinetics
Chemical Kineticsjc762006
╠²
This document summarizes key concepts in chemical kinetics including:
1) Kinetics is the study of reaction rates and how the molecular mechanism influences the rate. Factors like temperature, concentration, and catalysts affect the reaction rate.
2) The rate of a reaction is defined as the change in concentration of a reactant or product over time. Rate laws relate the reaction rate to concentrations of reactants.
3) Integrated rate laws allow calculation of reactant/product concentration as a function of time for different reaction orders (zero, first, second order). Graphical methods using these relations can determine the reaction order and rate constant.chemical-kinetics-ppt
chemical-kinetics-pptDaizyDmello2
╠²
This document provides an overview of chemical kinetics, including:
- Reaction rates are determined by how concentrations of reactants and products change over time.
- The rate law expresses the relationship between reaction rate and concentrations of reactants. Rate laws are determined experimentally.
- Reactions can be zero-order, first-order, or second-order depending on how the rate depends on concentrations.
- Reaction mechanisms involve elementary steps that describe reactions at the molecular level.
- Catalysts increase reaction rates by lowering the activation energy without being consumed in the process.Rate of chemical reaction
Rate of chemical reactionRAJEEVBAYAN1
╠²
Here are the steps to determine the order of the reaction:
1) Plot [X] vs time on a graph. You will get a straight line through the origin, indicating the reaction is first order.
2) Take the log of both sides of the rate law equation:
Rate = k[X]
Log(Rate) = Log(k[X])
3) Plot log(Rate) vs log([X]). You will get a straight line with a slope of 1, confirming the reaction is first order.
Therefore, based on the experimental data and analysis, this reaction is first order with respect to X.Kinetics ppt
Kinetics pptekozoriz
╠²
This document provides an overview of chemical kinetics and reaction rates. It discusses topics such as reaction rate, rate laws, reaction orders, rate constants, factors that affect reaction rates like temperature, catalysts, and enzyme kinetics. Specific examples are also provided to illustrate concepts like first-order and second-order reactions, reaction mechanisms, and industrial catalytic processes like the Haber process and catalytic converters.Kinetics.pptx
Kinetics.pptxFatmaMoustafa6
╠²
Chemical kinetics is the study of reaction rates and mechanisms. Key aspects include determining how factors like temperature, pressure, catalysts and light influence reaction rates. Reaction rates are determined by monitoring changes in reactant or product concentration over time. The rate of a reaction depends on the concentrations of reactants and can be modeled using a rate law. Common reaction orders include zero-order, first-order and second-order reactions, which have different relationships between rate and concentration. Catalysts increase reaction rates by providing an alternative reaction pathway with a lower activation energy.AP Chemistry Chapter 14 Powerpoint for website.ppt
AP Chemistry Chapter 14 Powerpoint for website.pptKimberlyAnnePagdanga1
╠²
This document summarizes key concepts in chemical kinetics. It discusses factors that affect reaction rates such as concentration, temperature, and catalysts. Reaction rates are expressed using rate laws and rate constants. Rate laws are determined experimentally and show how reaction rates depend on concentrations. Rates change over time and can be modeled using integrated rate laws for first and second order reactions. Reaction mechanisms involve elementary steps and may have intermediates. The activation energy required to reach the transition state affects temperature dependence of reaction rates.Chapter 17.4 : Reaction Rate
Chapter 17.4 : Reaction RateChris Foltz
╠²
This document provides information about chemical kinetics and reaction rates. It defines chemical kinetics and discusses the two conditions needed for reactions to occur: contact between particles and enough energy for activation. Reaction rate depends on collision frequency and efficiency of reactants and is influenced by nature of reactants, surface area, temperature, concentration, and presence of a catalyst. It also defines catalysts and different types. The relationship between reaction rate and concentration is given by the rate law, and order of a reaction relates to its rate law. Rate laws are written for different reaction examples and mechanisms.Chemical kinetics
Chemical kineticsDrgholamabbasChehard
╠²
1) This document summarizes key concepts from a lecture on chemical kinetics including reaction rates, rate laws, reaction mechanisms, and a collision model for chemical reactions.
2) Reaction rates describe how fast concentrations of reactants and products change over time. Rate laws define the relationship between reaction rate and concentrations of reactants.
3) Reaction mechanisms involve elementary steps that may involve intermediates to ultimately form products. The rate-determining step controls the overall rate.Chapter 12 chemical kinetics2
Chapter 12 chemical kinetics2DrgholamabbasChehard
╠²
1) This document summarizes key concepts from a lecture on chemical kinetics including reaction rates, rate laws, reaction mechanisms, and a collision model for chemical reactions.
2) Reaction rates describe how fast concentrations of reactants and products change over time. Rate laws define the relationship between reaction rate and concentrations of reactants.
3) Reaction mechanisms involve elementary steps that may involve intermediates to ultimately form products. The rate-determining step controls the overall rate.Chemical Kinetics chemical kinetics chemistry.ppt
Chemical Kinetics chemical kinetics chemistry.pptFatmaMoustafa6
╠²
Chemical Kinetics chemical kinetics chemistry.pptOrder of reaction and rate of reaction
Order of reaction and rate of reactionAhmad Hassan
╠²
The document discusses reaction order and methods for determining reaction order. It defines reaction order as the power dependence of the rate of reaction on the concentrations of reactants. Reaction order can be determined using the initial rates method, integral method, or differential method. Different reaction orders are also described, including zero-order reactions, where concentration does not affect rate; first-order reactions, where rate depends on one reactant; and second-order reactions, where rate depends on two reactants or one reactant squared. Pseudo-first order reactions are also discussed.Abc Kinetics
Abc Kineticsdluetgens
╠²
The document discusses kinetics and reaction rates. It defines kinetics as the study of reaction rates, and explains that thermodynamics determines if a reaction will occur but not how fast. It then covers factors that affect reaction rates like temperature, concentration, and catalysts. The document provides examples of calculating rates from concentration data and determining rate laws from experimental results. It also introduces integrated rate laws and how to determine reaction order from different rate law equations.Chemistry Lec-18A-19AB.pptx
Chemistry Lec-18A-19AB.pptxabdulahad563527
╠²
The document discusses reaction rates and orders. It states that the rate law can be used to calculate reaction rates if the rate constant (k), reaction order (x and y), and reactant concentrations are known. The overall reaction order is the sum of the powers of the reactant concentrations in the rate law. Reaction orders must be determined experimentally and cannot be deduced from the balanced reaction equation alone. Graphical methods can be used to determine the reaction order based on the shape of plots of concentration vs. time.More from Dr Nirankar Singh (6)
kinetics-lecture6.pdf
kinetics-lecture6.pdfDr Nirankar Singh
╠²
This document discusses key concepts relating to reaction rates:
- The half-life of a first-order reaction does not depend on initial concentration, while half-lives of second and zero-order reactions do depend on initial concentration.
- Reaction rates increase with higher concentrations because there are more collisions between reactant particles.
- Temperature also affects reaction rates because it increases the kinetic energy of particles, resulting in more frequent collisions exceeding the activation energy.
- The Arrhenius equation quantifies the exponential relationship between temperature and the rate constant.kinetics-lecture5.pdf
kinetics-lecture5.pdfDr Nirankar Singh
╠²
The document discusses reaction order and integrated rate laws for chemical reactions. It provides examples of determining the reaction order from concentration-time data and calculating rate constants from half-life. Specifically:
- The reaction order for N2O5 decomposition is determined to be first-order from a plot of ln[N2O5] vs. time.
- Integrated rate laws are presented for zero-order, first-order, and second-order reactions relating the change in concentration of a reactant over time.
- Half-life is defined as the time for a reactant concentration to reduce to half its initial value, and the equation to calculate half-life from the rate constant is given for firstkinetics-lecture4.pdf
kinetics-lecture4.pdfDr Nirankar Singh
╠²
This document discusses methods for determining the kinetics of chemical reactions, including:
1) Plotting concentration versus time data and determining reaction orders by examining how initial rates change with varying concentrations of reactants.
2) Using integrated rate laws - first-order reactions follow ln[A] vs. t, second-order 1/[A] vs. t, and zero-order [A] vs. t - to determine the rate constant k from the slopes of such plots.
3) An example problem demonstrates using the first-order integrated rate law to find product concentration after a time for a reaction.kinetics-lecture2.pdf
kinetics-lecture2.pdfDr Nirankar Singh
╠²
This document discusses rate laws and reaction orders. It contains the following key points:
- Rate laws express the rate of a reaction in terms of the concentrations of reactants and their change over time. For the reaction 2H2 + O2 ŌåÆ 2H2O, the rate is equal to the negative rate of change of the O2 concentration divided by two.
- Reaction orders describe how the rate of a reaction depends on the concentrations of reactants. A reaction can have different orders with respect to each reactant. First-order reactions have rates that change linearly with concentration on plots of concentration versus time or rate versus concentration.
- Common reaction orders are zero order, first order, and second order. Deterkinetics-lecture1.pdf
kinetics-lecture1.pdfDr Nirankar Singh
╠²
The document discusses factors that influence chemical reaction rates, including concentration of reactants, physical state of reactants, temperature, and surface area. It provides examples of how increasing concentration and temperature increase reaction rate by allowing more collisions. The document also discusses how reaction rate is measured based on changes in reactant or product concentration over time and presents data on the concentration of O3 at different times in its reaction with C2H4.Analytical Chemistry Introduction
Analytical Chemistry IntroductionDr Nirankar Singh
╠²
Analytical chemistry deals with determining the chemical composition of samples through qualitative and quantitative analysis. It has applications in fields like environmental science, clinical chemistry, and forensic science. Instrumental methods like spectroscopy, chromatography, and electroanalytical techniques are now commonly used for separation and analysis. These methods measure properties of samples like light absorption, emission spectra, electrical conductivity or potential to determine composition. Classical wet chemical methods like titration and gravimetry are also still used. Proper sampling, sample preparation, and control of instrumental factors are important for obtaining accurate results.Ad
Recently uploaded (20)
Sustainable Innovation with Immersive Learning
Sustainable Innovation with Immersive LearningLeonel Morgado
╠²
Prof. Leonel and Prof. Dennis approached educational uses, practices, and strategies of using immersion as a lens to interpret, design, and planning educational activities in a sustainable way. Rather than one-off gimmicks, the intent is to enable instructors (and institutions) to be able to include them in their regular activities, including the ability to evaluate and redesign them.
Immersion as a phenomenon enables interpreting pedagogical activities in a learning-agnostic way: you take a stance on the learning theory to follow, and leverage immersion to envision and guide your practice.Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...
Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...RAKESH SAJJAN
╠²
This PowerPoint presentation is based on Unit 4 ŌĆō Nutrition Assessment and Nutrition Education, a core topic in the 5th Semester of B.Sc Nursing under the subject Community Health Nursing ŌĆō I, as per the Indian Nursing Council (INC) guidelines.
The unit provides foundational knowledge of nutritional assessment techniques, importance of balanced diets, and health education strategies aimed at improving community nutrition. It empowers future nurses to play a key role in promoting nutrition, preventing malnutrition, and implementing dietary interventions at individual, family, and community levels.
Ō£ģ The PPT covers the following topics in detail:
Introduction to Nutrition and its role in health and disease prevention
Objectives of nutritional assessment in community settings
Types of nutritional assessment ŌĆō Anthropometric, Biochemical, Clinical, and Dietary (ABCD) methods
Tools and techniques used in each type of nutritional assessment
Interpreting growth charts, BMI, MUAC, and dietary recalls
Identification of malnutrition, both undernutrition and overnutrition
Common nutritional deficiencies ŌĆō protein-energy malnutrition, anemia, vitamin A deficiency, iodine deficiency
Principles of nutrition education and behavior change communication
Role of community health nurse in nutrition education during home visits, camps, and school health programs
Use of charts, posters, flashcards, and food models in health teaching
Culturally appropriate and locally available food suggestions
Strategies for promoting infant and young child feeding (IYCF)
National nutrition programs: POSHAN Abhiyaan, Mid-Day Meal Scheme, ICDS, and Anemia Mukt Bharat
Monitoring and evaluation of nutrition interventions
This PPT is perfect for:
B.Sc Nursing students preparing for unit tests or university exams
Nursing educators delivering community health lessons
Field work, community posting presentations, or group health teaching
Health educators and ASHA trainers working on community nutrition
All content is student-friendly, professionally formatted, and aligned with public health priorities and practical nursing roles.2025 June Year 9 Presentation: Subject selection.pptx
2025 June Year 9 Presentation: Subject selection.pptxmansk2
╠²
2025 June Year 9 Presentation: Subject selectionEnvironmental Science, Environmental Health, and Sanitation ŌĆō Unit 3 | B.Sc N...
Environmental Science, Environmental Health, and Sanitation ŌĆō Unit 3 | B.Sc N...RAKESH SAJJAN
╠²
This PowerPoint presentation covers Unit 3 ŌĆō Environmental Science, Environmental Health, and Sanitation from the 5th Semester B.Sc Nursing syllabus prescribed by the Indian Nursing Council (INC). It is carefully designed to support nursing students, educators, and community health professionals in understanding the environmental components that influence health and disease prevention.
The unit emphasizes the interrelationship between the environment and human health, highlighting various environmental factors, hazards, and strategies for disease prevention through sanitation and public health initiatives.
Ō£│’ĖÅ Topics Covered in the PPT:
Definition and scope of environmental science and environmental health
Importance of a safe environment for public health
Types of environmental pollution ŌĆō air, water, soil, noise, and radiation
Sources, effects, and prevention of different types of pollution
Concept of ecosystem and its components
Water safety and purification methods at household and community levels
Disposal of waste and excreta ŌĆō types, methods, health risks
Introduction to environmental sanitation
Vector control measures: Mosquitoes, houseflies, rodents, etc.
Biological and non-biological health hazards in the environment
National programs related to environmental health and sanitation
Health education for safe water, hygiene, and sanitation behavior change
Role of a community health nurse in promoting environmental health
Use of community bags and home visit kits to educate rural families
Practical methods for solid waste management and waste segregation
This presentation supports:
Class lectures and revision
Health teaching in field visits
Community awareness campaigns
Internal assessments and final exam preparation
It ensures that all essential environmental health concepts are simplified and well-structured for easy understanding and application in nursing practice.YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
BLUF:
The Texas outbreak has slowed down, but sporadic cases continue to emerge in Kansas, Oklahoma, and New Mexico.
Elsewhere in the US, we continue to see signs of acceleration due to outbreaks outside the Southwest (North Dakota, Montana, and Colorado) and travel-related cases. Measles exposures due to travel are expected to pose a significant challenge throughout the summer.
The U.S. is on track to exceed its 30-year high for measles cases (1,274) within the next two weeks.
Here is the latest update:
CURRENT CASE COUNT: 919
ŌĆóTexas: 744 (+2) (55% of cases are in Gaines County).
ŌĆóNew Mexico: 81 (83% of cases are from Lea County).
ŌĆóOklahoma: 20 (+2)
ŌĆóKansas: 74 (+5) (38.89% of the cases are from Gray County).
HOSPITALIZATIONS: 104
ŌĆó Texas: 96 (+2) ŌĆō This accounts for 13% of all cases in Texas.
ŌĆó New Mexico: 7 ŌĆō This accounts for 9.47% of all cases in New Mexico.
ŌĆó Kansas: 3 ŌĆō This accounts for 5.08% of all cases in the state of Kansas.
DEATHS: 3
ŌĆóTexas: 2 ŌĆō This is 0.27% of all cases in Texas.
ŌĆóNew Mexico: 1 ŌĆō This is 1.23% of all cases in New Mexico.
US NATIONAL CASE COUNT: 1,197
INTERNATIONAL SPREAD
ŌĆóMexico: 2337 (+257), 5 fatalities
ŌĆÆChihuahua, Mexico: 2,179 (+239) cases, 4 fatalities, 7 currently hospitalized.
ŌĆóCanada: 3,207 (+208), 1 fatality
ŌĆÆOntario Outbreak, Canada: 2,115 (+74) cases, 158 hospitalizations, 1 fatality.
ŌĆÆAlberta, Canada: 879(+118) cases, 5 currently hospitalized.ECONOMICS, DISASTER MANAGEMENT, ROAD SAFETY - STUDY MATERIAL [10TH]
ECONOMICS, DISASTER MANAGEMENT, ROAD SAFETY - STUDY MATERIAL [10TH]SHERAZ AHMAD LONE
╠²
This study material for Class 10th covers the core subjects of Economics, Disaster Management, and Road Safety Education, developed strictly in line with the JKBOSE textbook. It presents the content in a simplified, structured, and student-friendly format, ensuring clarity in concepts. The material includes reframed explanations, flowcharts, infographics, and key point summaries to support better understanding and retention. Designed for classroom teaching and exam preparation, it aims to enhance comprehension, critical thinking, and practical awareness among students.Code Profiling in Odoo 18 - Odoo 18 ║▌║▌▀Żs
Code Profiling in Odoo 18 - Odoo 18 ║▌║▌▀ŻsCeline George
╠²
Profiling in Odoo identifies slow code and resource-heavy processes, ensuring better system performance. Odoo code profiling detects bottlenecks in custom modules, making it easier to improve speed and scalability.This is why students from these 44 institutions have not received National Se...
This is why students from these 44 institutions have not received National Se...Kweku Zurek
╠²
This is why students from these 44 institutions have not received National Service PIN codes (LIST)Tanja Vujicic - PISA for Schools contact Info
Tanja Vujicic - PISA for Schools contact InfoEduSkills OECD
╠²
Tanja Vujicic, Senior Analyst and PISA for SchoolŌĆÖs Project Manager at the OECD spoke at the OECD webinar 'Turning insights into impact: What do early case studies reveal about the power of PISA for Schools?' on 20 June 2025
PISA for Schools is an OECD assessment that evaluates 15-year-old performance on reading, mathematics, and science. It also gathers insights into studentsŌĆÖ learning environment, engagement and well-being, offering schools valuable data that help them benchmark performance internationally and improve education outcomes. A central ambition, and ongoing challenge, has been translating these insights into meaningful actions that drives lasting school improvement. How to Manage Different Customer Addresses in Odoo 18 Accounting
How to Manage Different Customer Addresses in Odoo 18 AccountingCeline George
╠²
A business often have customers with multiple locations such as office, warehouse, home addresses and this feature allows us to associate with different addresses with each customer streamlining the process of creating sales order invoices and delivery orders.Communicable Diseases and National Health Programs ŌĆō Unit 9 | B.Sc Nursing 5t...
Communicable Diseases and National Health Programs ŌĆō Unit 9 | B.Sc Nursing 5t...RAKESH SAJJAN
╠²
This PowerPoint presentation covers Unit 9 ŌĆō Communicable Diseases and National Health Programs, a core part of the 5th Semester B.Sc Nursing (Community Health Nursing ŌĆō I) syllabus, as outlined by the Indian Nursing Council (INC).
This unit enables nursing students to understand the epidemiology, prevention, control, and nursing management of common communicable diseases in India, while also offering a structured overview of the National Health Programs implemented to address them.
The content is critical for effective field practice, disease surveillance, early detection, referral, and health education, equipping students to participate in public health interventions and outbreak control at community and national levels.
¤ōś Key Topics Covered in the PPT:
Definition and classification of communicable diseases
Modes of transmission and chain of infection
Common communicable diseases in India:
Malaria
Tuberculosis
Leprosy
Dengue
HIV/AIDS
Hepatitis
COVID-19 (if included in the current curriculum)
Diarrheal diseases
Acute Respiratory Infections (ARIs)
Epidemiological factors, causative agents, symptoms, and incubation periods
Prevention and control strategies: primary, secondary, and tertiary levels
Nursing responsibilities in patient care, contact tracing, community surveillance, and outbreak control
Health education and behavior change communication for community awareness
Vaccination schedules and cold chain maintenance
National Health Programs related to communicable diseases:
National Vector Borne Disease Control Program (NVBDCP)
Revised National Tuberculosis Control Program (RNTCP)
National Leprosy Eradication Program (NLEP)
National AIDS Control Program (NACP)
Universal Immunization Program (UIP)
IDSP ŌĆō Integrated Disease Surveillance Program
Overview of standard treatment protocols, referral mechanisms, and community nurseŌĆÖs role in program implementation
This presentation is ideal for:
Nursing students preparing for university exams, class tests, and field projects
Tutors teaching infectious disease nursing and public health interventions
Nurses involved in immunization, outbreak investigation, and contact tracing
It provides a student-friendly breakdown of concepts, aligned with national priorities, including flowcharts, tables, case examples, and simplified text for field-level application.Chalukyas of Gujrat, Solanki Dynasty NEP.pptx
Chalukyas of Gujrat, Solanki Dynasty NEP.pptxDr. Ravi Shankar Arya Mahila P. G. College, Banaras Hindu University, Varanasi, India.
╠²
This presentation has been made keeping in mind the students of undergraduate and postgraduate level. In this slide try to present the brief history of Chaulukyas of Gujrat up to Kumarpala To keep the facts in a natural form and to display the material in more detail, the help of various books, websites and online medium has been taken. Whatever medium the material or facts have been taken from, an attempt has been made by the presenter to give their reference at the end.
Chaulukya or Solanki was one of the Rajputs born from Agnikul. In the Vadnagar inscription, the origin of this dynasty is told from Brahma's Chauluk or Kamandalu. They ruled in Gujarat from the latter half of the tenth century to the beginning of the thirteenth century. Their capital was in Anahilwad. It is not certain whether it had any relation with the Chalukya dynasty of the south or not. It is worth mentioning that the name of the dynasty of the south was 'Chaluky' while the dynasty of Gujarat has been called 'Chaulukya'. The rulers of this dynasty were the supporters and patrons of Jainism.Plate Tectonic Boundaries and Continental Drift Theory
Plate Tectonic Boundaries and Continental Drift TheoryMarie
╠²
This 28 slide presentation covers the basics of plate tectonics and continental drift theory. It is an effective introduction into a full plate tectonics unit study, but does not cover faults, stress, seismic waves, or seafloor spreading.
To download PDF, visit The Homeschool Daily. We will be uploading more slideshows to follow this one. Blessings, Marie Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
Dive into a captivating analysis where Kazuo IshiguroŌĆÖs nuanced fiction meets the stark realities of postŌĆæ2014 Indian journalism. Uncover how ŌĆ£Godi MediaŌĆØ turned from watchdog to lapdog, echoing the moral compromises of IshiguroŌĆÖs protagonists. WeŌĆÖll draw parallels between restrained narrative silences and sensationalist headlinesŌĆöare our media heroes or traitors? DonŌĆÖt forget to follow for more deep dives!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 107: The Twentieth Century Literature: From World War II to the End of the Century
Submitted Date: April 4, 2025
Paper Name: The Twentieth Century Literature: From World War II to the End of the Century
Topic: From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi MediaŌĆØ in Post-2014 Indian Journalism
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/kIEqwzhHJ54
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/from-watchdog-to-lapdog-ishiguro-s-fiction-and-the-rise-of-godi-media-in-post-2014-indian-journalism.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#GodiMedia #Ishiguro #MediaEthics #WatchdogVsLapdog #IndianJournalism #PressFreedom #LiteraryCritique #AnArtistOfTheFloatingWorld #MediaCapture #KazuoIshiguro
Keyword Tags:
Godi Media, Ishiguro fiction, post-2014 Indian journalism, media capture, Kazuo Ishiguro analysis, watchdog to lapdog, press freedom India, media ethics, literature and media, An Artist of the Floating WorldBattle of Bookworms 2025 - U25 Literature Quiz by Pragya
Battle of Bookworms 2025 - U25 Literature Quiz by Pragya Pragya - UEM Kolkata Quiz Club
╠²
Battle of Bookworms is a literature quiz organized by Pragya, UEM Kolkata, as part of their cultural fest Ecstasia. Curated by quizmasters Drisana Bhattacharyya, Argha Saha, and Aniket Adhikari, the quiz was a dynamic mix of classical literature, modern writing, mythology, regional texts, and experimental literary forms. It began with a 20-question prelim round where ŌĆśstar questionsŌĆÖ played a key tie-breaking role. The top 8 teams moved into advanced rounds, where they faced audio-visual challenges, pounce/bounce formats, immunity tokens, and theme-based risk-reward questions. From Orwell and Hemingway to Tagore and Sarala Das, the quiz traversed a global and Indian literary landscape. Unique rounds explored slipstream fiction, constrained writing, adaptations, and true crime literature. It included signature IDs, character identifications, and open-pounce selections. Questions were crafted to test contextual understanding, narrative knowledge, and authorial intent, making the quiz both intellectually rewarding and culturally rich. Battle of Bookworms proved literature quizzes can be insightful, creative, and deeply enjoyable for all.Publishing Your Memoir with Brooke Warner
Publishing Your Memoir with Brooke WarnerBrooke Warner
╠²
Brooke Warner presents on getting published - traditional, hybrid, and self-publishing.
www.memoirnation.comYSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
Chalukyas of Gujrat, Solanki Dynasty NEP.pptx
Chalukyas of Gujrat, Solanki Dynasty NEP.pptxDr. Ravi Shankar Arya Mahila P. G. College, Banaras Hindu University, Varanasi, India.
╠²
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
Ad
kinetics-lecture3.pdf
- 1. Individual and Overall Reaction Orders For the reaction 2NO(g) + 2H2(g) ŌåÆ N2(g) + 2H2O(g): The rate law is rate = k[NO]2[H2] The reaction is second order with respect to NO, first order with respect to H2 and third order overall. Note that the reaction is first order with respect to H2 even though the coefficient for H2 in the balanced equation is 2. Reaction orders must be determined from experimental data and cannot be deduced from the balanced equation. Dr. N. Singh
- 2. PLAN: We inspect the exponents in the rate law, not the coefficients of the balanced equation, to find the individual orders. We add the individual orders to get the overall reaction order. SOLUTION: (a) The exponent of [NO] is 2 and the exponent of [O2] is 1, so the reaction is second order with respect to NO, first order with respect to O2 and third order overall. Determining Reaction Orders from Rate Laws PROBLEM2: For each of the following reactions, use the give rate law to determine the reaction order with respect to each reactant and the overall order. (a) 2NO(g) + O2(g) ŌåÆ 2NO2(g); rate = k[NO]2[O2] (b) CH3CHO(g) ŌåÆ CH4(g) + CO(g); rate = k[CH3CHO]3/2 (c) 3 H2O2(aq) + 3I-(aq) + 2H+(aq) ŌåÆI -(aq) + 2H2O(l); rate = k[H2O2][I-] Dr. N. Singh
- 3. Problem 2 (b) The reaction is 3 order in CH3CHO and 3 order overall. 2 2 (c) The reaction is first order in H2O2, first order in I-, and second order overall. The reactant H+ does not appear in the rate law, so the reaction is zero order with respect to H+. Dr. N. Singh
- 4. Determining Reaction Orders For the general reaction A + 2B ŌåÆ C +D, the rate law will have the form Rate = k[A]m[B]n Todetermine the values of m and n, we run a series of experiments in which one reactant concentration changes while the other is kept constant, and we measure the effect on the initial rate in each case. Dr. N. Singh
- 5. Table 2 Initial Rates for the Reaction between A and B Initial Rate Initial [A] Initial [B] Experiment (mol/L┬Ęs) (mol/L) (mol/L) 1 1.75x10-3 2.50x10-2 3.00x10-2 2 3.50x10-3 5.00x10-2 3.00x10-2 3 3.50x10-3 2.50x10-2 6.00x10-2 4 7.00x10-3 5.00x10-2 6.00x10-2 [B] is kept constant for experiments 1 and 2, while [A] is doubled. Then [A] is kept constant while [B] is doubled. Dr. N. Singh
- 6. Rate 2 Rate 1 = k[A] m [B]n 2 2 1 k[A] m [B]n 1 Finding m, the order with respect to A: We compare experiments 1 and 2, where [B] is kept constant but [A] doubles: = [A] m 2 m [A]1 = [A]2 m 3.50x10-3 mol/L┬Ęs 1.75x10-3mol/L┬Ęs = 5.00x10-2 mol/L 2.50x10-2 mol/L [A]1 m Dividing, we get 2.00 = (2.00)m so m = 1 Dr. N. Singh
- 7. Rate 3 Rate 1 = k[A] m [B]n 3 3 1 k[A] m [B]n 1 Finding n, the order with respect to B: We compare experiments 3 and 1, where [A] is kept constant but [B] doubles: = [B] n 3 n [B]1 = [B]3 n 3.50x10-3 mol/L┬Ęs 1.75x10-3mol/L┬Ęs = 6.00x10-2 mol/L 3.00x10-2 mol/L [B]1 m Dividing, we get 2.00 = (2.00)n so n = 1 Dr. N. Singh
- 8. Table 3 Initial Rates for the Reaction between O2 and NO O2(g) + 2NO(g) ŌåÆ 2NO2(g) Rate = k[O2]m[NO]n Initial Rate Initial Reactant Concentrations (mol/L) Experiment (mol/L┬Ęs) [O2] [NO] 1 3.21x10-3 1.10x10-2 1.30x10-2 2 6.40x10-3 2.20x10-2 1.30x10-2 3 12.48x10-3 1.10x10-2 2.60x10-2 4 9.60x10-3 3.30x10-2 1.30x10-2 5 28.8x10-3 1.10x10-2 3.90x10-2 Dr. N. Singh
- 9. Rate 2 Rate 1 = 2 2 2 1 k[O ] m [NO]n 1 Finding m, the order with respect to O2: We compare experiments 1 and 2, where [NO] is kept constant but [O2] doubles: = k[O2] m [NO]n [O2] m [O ]m 2 1 2 = [O ] 2 2 [O ] 2 1 m 6.40x10-3 mol/L┬Ęs = 2.20x10-2 mol/L 3.21x10-3mol/L┬Ęs 1.10x10-2 mol/L Dividing, we get 1.99 = (2.00)m or 2 = 2m, so m = 1 The reaction is first order with respect to O2. m Dr. N. Singh
- 10. Sometimes the exponent is not easy to find by inspection. In those cases, we solve for m with an equation of the form a = bm: log a = log 1.99 log b log 2.00 m = = 0.993 This confirms that the reaction is first order with respect to O2. Reaction orders may be positive integers, zero, negative integers, or fractions. Dr. N. Singh
- 11. Finding n, the order with respect to NO: We compare experiments 1 and 3, where [O2] is kept constant but [NO] doubles: Rate 3 = [NO]3 Rate 1 [NO]1 n = 12.8x10-3 mol/L┬Ęs 2.60x10-2 mol/L 3.21x10-3mol/L┬Ęs 1.30x10-2 mol/L n Dividing, we get 3.99 = (2.00)n or 4 = 2n, so n =2. The reaction is second order with respect to NO. The rate law is given by: rate = k[O2][NO]2 log a = log 3.99 log b log 2.00 n = = 2.00 Alternatively: Dr. N. Singh
- 12. Sample Problem 3 Determining Reaction Orders from Rate Data PROBLEM: Many gaseous reactions occur in a car engine and exhaust system. One of these reactions is NO2(g) + CO(g) ŌåÆ NO(g) + CO2(g) rate = k[NO2]m[CO]n Use the following data to determine the individual and overall reaction orders: Experiment Initial Rate (mol/L┬Ęs) Initial [NO2] (mol/L) Initial [CO] (mol/L) 1 0.0050 0.10 0.10 2 0.080 0.40 0.10 3 0.0050 0.10 0.20 Dr. N. Singh
- 13. Sample Problem 3 PLAN: We need to solve the general rate law for m and for n and then add those orders to get the overall order. We proceed by taking the ratio of the rate laws for two experiments in which only the reactant in question changes concentration. rate 2 rate 1 [NO ] 2 1 k [NO ]m [CO]n [NO ] 2 2 2 = 2 2 1 k [NO2]m [CO]n 1 = 0.080 0.40 0.0050 0.10 = m SOLUTION: Tocalculate m, the order with respect to NO2, we compare experiments 1 and 2: m 16 = (4.0)m so m = 2 The reaction is second order in NO2. Dr. N. Singh
- 14. k [NO ]m [CO]n [CO] 3 [CO]1 2 3 3 = k [NO2]m [CO]n 1 1 n rate 3 rate 1 = = 0.0050 0.20 0.0050 0.10 n 1.0 = (2.0)n so n = 0 The reaction is zero order in CO. rate = k[NO2]2[CO]0 or rate = k[NO2]2 Sample Problem 3 Tocalculate n, the order with respect to CO, we compare experiments 1 and 3: Dr. N. Singh
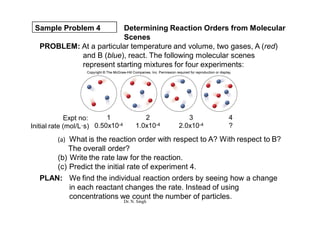
- 15. Sample Problem 4 Determining Reaction Orders from Molecular Scenes PROBLEM: At a particular temperature and volume, two gases, A (red) and B (blue), react. The following molecular scenes represent starting mixtures for four experiments: (a) What is the reaction order with respect to A? With respect to B? The overall order? (b) Write the rate law for the reaction. (c) Predict the initial rate of experiment 4. PLAN: We find the individual reaction orders by seeing how a change in each reactant changes the rate. Instead of using concentrations we count the number of particles. Expt no: Initial rate (mol/L┬Ęs) 1 0.50x10-4 2 1.0x10-4 3 2.0x10-4 4 ? Dr. N. Singh
- 16. Sample Problem 4 SOLUTION: (a) For reactant A(red): Experiments 1 and 2 have the same number of particles of B, but the number of particles of A doubles. The rate doubles. Thus the order with respect to A is 1. For reactant B (blue): Experiments 1 and 3 show that when the number of particles of B doubles (while A remains constant), the rate quadruples. The order with respect to B is 2. The overall order is 1 + 2 = 3. (b) Rate = k[A][B]2 (c) Between experiments 3 and 4, the number of particles of A doubles while the number of particles of B does not change. The rate should double, so rate = 2 x 2.0x10-4 = 4.0x10-4mol/L┬Ęs Dr. N. Singh
