Lab Connect Overview.2011 03 29
- 1. “Tłó±đ world’s local central lab. Global reach. Local expertise.” 1
- 2. LabConnect Overview • Global laboratory services organization supporting studies across six continents • Central laboratory services – Worldwide testing for clinical studies – Project and data management QuickTime™ and a decompressor – Supplies and logistics are needed to see this picture. • Specialized laboratory services – Leverage network to design solutions to match client needs – Focus on specialized analysis and external R&D project management 2
- 3. LabConnect Overview- Experience • Approximately 340 contracted studies since company’s inception in late 2002. • 80-90 new projects per year. • Diverse client base • Customized service solutions • Comprehensive Laboratory Project Management • Client Service/Follow Up/Flexibility 3
- 4. Organizational Design • Foundation Concepts- Business Model – Service Orientation (Project Management, Data Delivery, Site Support) – Geographic Reach – Analytical Diversity • LabConnect study support team – Conduct all pre/post analytical functions. – Project management – Data management – Reporting – Logistic and supply coordination – Training and site support • Worldwide network of laboratories selected for their clinical research experience, geographic locations and analytical expertise. 4
- 5. Global Locations Central Laboratory Locations-Primary • North America-Johnson City, TN • Europe-Gdansk, Poland • Asia-Mumbai, India • Asia-Guangzhou, China Central Laboratory Locations-Regional • Europe-Bucharest, Romania • Europe-Kiev, Ukraine • Europe-Minsk, Belarus • Europe-Tbilisi, Georgia • Asia-Seoul, South Korea • Asia-Tel Aviv, Israel • Asia-Istanbul, Turkey • Africa- Cape Town, SA • Australia-Melbourne • Australia-Sydney • South America-Buenos Aires Specialized Laboratories (15 labs) • Platform expertise • Therapeutic area • Analysis category Corporate Offices • Seattle, WA 5
- 6. Analytical Capabilities • Routine and Specialized Laboratory Testing • Molecular Diagnostics • Oncology • Flow Cytometry • Molecular Oncology • Immunohistochemistry • Cytogenetics • Circulating Tumor Cells • Microbiology • Multiplex Assay • ELISA • Analytical Chemistry • Immunoassays • Immunogenicity assays • Method Development Services • Anatomic Pathology • Biostorage 6
- 7. Network Standards and Harmonization • Participating Laboratories all have region specific accreditations including CAP, CLIA, ISO 17025 • Proficiency testing • Track record of successful audits by Sponsors and CRO’s • Qualification Process • Internal audits • Intra-laboratory comparison studies
- 8. Experience by Therapeutic Area Women's Health Cardiology 6% 9% CNS 11% Oncology 23% Dermatology 16% Misc Endocrinology 20% GI 4% Infectious Disease 4% 8%
- 9. Competitive Advantages • Custom service plans for each project • Coordination of all laboratory requirements – Routine – Esoteric – Biomarker – Pharmacogenomic – Pharmacokinetics – Bio-storage • Analytical expertise – Selected for your project – Available across the globe • Innovative and flexible reporting technologies • Service focused organization • Regional expertise 9
- 10. Central Lab Services • Project Management • Investigator Site Support • Specimen Collection and Transport Supplies • Logistics • Reporting-ClinReach™ o Fax/Email/VPN/Web o TAT-Day of receipt • Data Management • Specimen Tracking and Management • Worldwide Biostorage 10
- 11. Specialized Services Support • Combines Scientific Project Management expertise with LabConnect network and infrastructure • Broad base of analytical capabilities and experience within network • Assay Development • Integration with LabConnect Project Management and Project Support 11
- 12. Project Management • A Project Manager will be assigned to your study • For global studies, regional project teams will support each unique geographic region • The Project Manager – Establish the standards for study documents – Coordinate activities across LabConnect Network – Provide ongoing support to your project • Participate in project team meetings • Monitor results and investigator site compliance • Ensure that deliverable dates are met. 12
- 13. LabConnect Project Management Coordination of Full Spectrum of Laboratory Services across Network Specimen Full Coordination of Routine Tracking Testing Data Management Kits Logistics Training Materials Sample Management Bioanalytical Esoteric Testing Testing Ongoing Site Support 13
- 14. Study Start Up – Complete-”Statement of Work” (SOW) – Study set up • Investigator Manual • LIS/IT Validation • Test Data Transfer • Requisitions • Kits to Sites – On average, initial shipments are delivered to sites within four to six weeks after SOW is completed. 14
- 15. Investigator Site Support • Training –Investigator Meetings • Off Site • Web-based – Comprehensive Laboratory Manual • Telephonic and email access to site support • Employee compensation is tied to investigator site satisfaction 15
- 16. Investigator Site Survey Results (Cumulative through September 2010) ACM Covance ICON LabConnect LabCorp Quest Quintiles Other % % % % % % % % Best Client Service Staff Total 3% 39% 10% 44% 5% 14% 14% 12% Best Courier Service Total 3% 36% 11% 33% 6% 26% 16% 17% Best Lab Kits Total 5% 41% 14% 39% 4% 13% 18% 18% Best Lab Reports Total 4% 37% 10% 41% 7% 17% 16% 11% Best Lab Requisition Forms Total 3% 40% 10% 38% 5% 16% 18% 4% Best Study Training & Manuals Total 3% 39% 12% 36% 5% 17% 20% 12% Best Overall Service Total 3% 37% 9% 41% 4% 16% 13% 10% 16
- 17. Custom Collection/Transport Supplies • Kits are custom designed by Project Management to meet your specific requirements • Each collection kit comes contained within the return shipper. • Tubes are pre-labeled to minimize errors • Pre-printed shipping labels • Kits are study/site/visit specific • Resupply requests will be fulfilled within 3-5 business days 17
- 21. Reporting-LabREACH Technology • Reports Include: – Abnormal and Flag Alerts – Historical values for out of range results – Custom calculations/reports • Same Day Delivery via LabREACH™: – LabREACH™ Suite • Direct • Online • AutoFax • Email 21
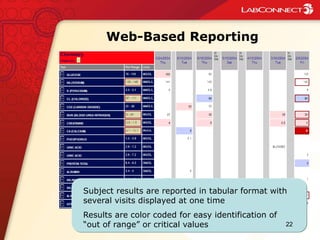
- 22. Web-Based Reporting Subject results are reported in tabular format with several visits displayed at one time Results are color coded for easy identification of “out of range” or critical values 22
- 23. Trending Reports 23
- 24. Trending Reports 24
- 25. Trending Reports 25
- 26. Trending Reports 26
- 27. Data Management The delivery of clean data throughout your study is achieved through the execution of basic processes. • Requisition Slips – Have easy to follow instructions – Protocol/Site/Visit specific – Bar-coded Accession Number • Pre-labeled collection tubes • Automated order entry system 27
- 28. Data Management/Data Delivery • Real time resolution of specimen, requisition, and demographic inconsistencies. • Project Management and/or Investigator Services will notify the site to resolve. • Daily order entry QC reports and resolution • Data can be transmitted according to your pre- defined schedule, monthly transfer is standard. • ASCII and CDISC data formats standard 28
- 29. Specimen Logistics Samples will be delivered from the sites to the laboratory within 24-36 hours FedEx and DHL are primary couriers Dry ice can be provided to the sites should they require. Delivery times will be monitored and corrective action taken as needed If needed, dry runs will be conducted to determine transport times and aid in courier selection 29
- 30. Sample Tracking and Management • LabConnect offers comprehensive menu specimen management services. • Tracking • Identification • Management • Storage 30
- 31. Current challenges in sample tracking • Increase in esoteric testing requires more detailed sample management • Difficult to get comprehensive sample reconciliation during on-going clinical trial – Sample tracking is often done manually by clinical operations group, requires extra man hours and results in higher error rate • Limited visibility at sites – Specimens can sit at sites for extended periods – No visibility until samples are shipped/received at a lab – Difficult to catch problems at a resolvable stage • Hard to monitor sample quality • Difficult to identify samples in system based on clinical criteria
- 32. There are gaps in current sample tracking systems Lab 1 Client or Sites Central lab Lab 2 storage Lab 3 Samples are not Labs (academia or Difficult to reconcile monitored until they are specialized labs) frequently multiple outputs from received at a lab or by the do not have automated different locations. client. sample management Increased error rate. capabilities
- 33. LabConnect sample tracking system Lab 1 Client or Sites LabConnect Lab 2 storage Lab 3 Database Digital pen technology and data integration tools allows instant access to sample information and comprehensive real time sample tracking.
- 34. Digital pen technology allows increased visibility without changing behavior 1 2 3 4 Sample Lab receives Sample sample and enters Collection shipped into LIMS Site documentation Sample stored, Site personnel batched shipped later Digital pen captures site documentation instantly, importing to database
- 35. Integration of various sample data files csv, excel, Sites xml, ascii, txt Labs csv, excel, xml, ascii, txt DB • Canned queries csv, excel, • Reports Storage xml, ascii, txt • Ask questions about the samples • Filter • Search
- 36. Case study: Sample tracking solution for large pharma company Lab1 Sample types: Lab2 Tumor Sites Client lab Cell pellet Lab3 Skin CTC Lab4 Client storage Hair Blood Lab5 Serum Plasma- 16 aliquots  Samples being tracked manually, by multiple people  Data was inconsistent, full of errors, not complete  Samples were unaccounted for  Difficult to determine number of missing samples
- 37. LabConnect System Benefits • Real time tracking, starting at the time of collection • Instant accessioning • Better control on shipping logistics • Fast identification and correction of queries/issues • Multiple user web access (smart phone application available) • Customizable reports • Solid chain of custody • Yields documented quality sample management and saves time 37
- 38. www.labconnectllc.com 38
Editor's Notes
- Add in some bullet points in previous slide… sites are manually tracking, no oversight, samples are sitting at site for unknown amounts of time, etc.