Lecture 0 introduction(3)
- 1. INTRODUCTION to MATERIAL AND ENERGY BALANCES By: Ms. Siti Nur Fadhilah Zainudin (Ms. Dilla) B.Eng. (Hons.) Biochemical Engineering M. Sc. Chemical Engineering (Nanoparticle Thin film) Email: fadhilah@ucsiuniversity.edu.my
- 2. Contents Time-table Course Outline Assessment Class Policy Assignment LMS & Facebook Page Back to Basics
- 3. Time-Table Lecture: Wednesday 9.30 – 11.00 am (K208) Friday 9.30 – 11.00 am (K208) Tutorial: to be notified Wed 3.30 – 5.00 pm (K211) Consultation hours: Upon request (Email: fadhilah@ucsiuniversity.edu.my)
- 4. Course Outline ÔÇó Topics Covered. ÔÇó Units and Dimensions ÔÇó Processes and Process Variables ÔÇó Material balance of non-reactive processes ÔÇó Material balance of reactive processes ÔÇó Energy balance of non-reactive processes ÔÇó Energy balance of non-reactive processes
- 5. Assessment Component Test(5/6/2015) 20% Mid-Term (3/7/2015) 20% Mini project/case study (10/6/2015) 10% Final Exam 50%
- 6. Course material  Main course reference/textbook: Felder, R. M. and Rousseau, R. W. (2000). Elementary Principles of Chemical Processes. John Wiley & Sons.  Supplementary text: Himmelblau, D. M., Riggs, J. B. (2004). Basic Principles and Calculations in Chemical Engineering, International Edition, 7th Edition, Prentice Hall International Series Get a copy of the text book from UCSI Bookstore.
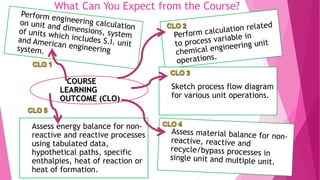
- 7. What Can You Expect from the Course? COURSE LEARNING OUTCOME (CLO) Sketch process flow diagram for various unit operations. Assess energy balance for non- reactive and reactive processes using tabulated data, hypothetical paths, specific enthalpies, heat of reaction or heat of formation.
- 8. Class Policy ÔÇó Be punctual to class. ÔÇó Late attendance (>30 minutes) will not be considered. ÔÇó No hand phones during class. ÔÇó Barred from final exam if miss 3 consecutive classes and/or less than 80% attendance (AUTO BARRING BY IIS). ÔÇó Barred from final exam if total internal marks less than 20/50. ÔÇó Attendance in tutorial sessions are COMPULSORY. ÔÇó MCs to be submitted to lecturer within 1 week from date of absent. ÔÇó No replacement for assessments unless with valid MC from government hospital only. ÔÇó 50% ruling for final exam.
- 9. LMS Code  JAVV – GXCM Learning Management System (LMS)
- 10. Back to Basics ÔÇó Ideal gas equation of state ÔÇó Chemical balance equation ÔÇó Study the concept of thermodynamics of process systems
- 11. Tips for Success: ÔÇó Revisit the lesson. ÔÇó Attempt tutorial questions by yourself. ÔÇó Practice, practice and more practice. ÔÇó Do not accumulate doubts. Ask! ÔÇó Do not be late for class! ÔÇó Study SMART ÔÇó Join PAL session ÔÇó Keep calm and confidence ÔÅä
- 12. GROUP ASSIGNMENT Student StudentID GROUP Ong Kwang Zhean 1001334006 1 Harriprashanth A/L Shanmugavel 1001438249 1 Loveroyce A/L Miclamani 1001437372 1 Sim Mei Ting 1001334333 2 Chan Kah Deng 1001437571 2 Lam Chi Hang 1001334299 2 Thi Shiki 1001438147 3 Jong Tzy Shi 1001436608 3 Alabi Hanif Odunayo 1001336024 3 Gan Jia Ler 1001436606 4 Logeswaran A/L Sinna Thaanby 1001438312 4 Yap Wei Lun 1001437889 4 Seng May Suet 1001333938 5 Chan Kok Keong 1001334024 5 Yap Khun Yee 1001438406 5 Sugheensan A/L Magesan 1001334690 5
- 13. GROUP ASSIGNMENT Chong Mei Mei 1001335184 6 Foo Mou Yang 1001334134 6 Lee Mei Yen 1001438530 6 Dineswari A/P Chandrasagaran 1001437636 6 Sai Yit Wen 1001438509 7 Keshuna Naidu A/P Uthamarajoo 1001438661 7 Janarthanan Rethi A/L Selva Raja 1001438514 7 Aaron Das Raju 1001232021 7 Ahalyah A/P Ragunathan 1001334724 8 Lee Chi Cheng 1001438596 8 Faisal Ahmed Bin Ab Shofi Ahammed 1001437528 8 Arwin A/L P Jayapalan 1001130397 8 Tan Ning, Jacqueline 1001233545 9 Yap Zjin Hung 1001334536 9 Ng Mun Hou 1001334210 9 Nisha A/P K.Arultharuman 1001437032 9
- 14. GROUP ASSIGNMENT Anusuyah A/P Ragunathan 1001334723 10 Wong Yew Mun 1001437602 10 Chan Jian Fu 1001333674 10 Asaad Fahad Hamdan Al-Housni 1001436502 10 Chow Kah Jun 1001438591 11 Lim Chun Wei 1001436665 11 Neoh Yu Xiang 1001438138 11 Chuah Tse Jun Eugene 1001334192 11 Lye Qing Yuan 1001438670 12 Toong Jin Fung 1001438272 12 Goh Chai Ni 1001231547 12 Grace Melona Antony Selvaraj 1001335924 12 Ng Choon Khon 1001438550 13 Chua Yuan Yuan 1001334132 13 Charndeep K. Singh Sandhu 1001437902 13 Anouar Adam Oumar Kharif 1001027576 13
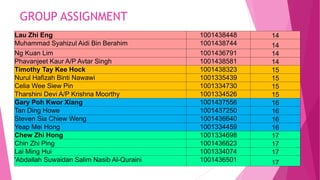
- 15. GROUP ASSIGNMENT Lau Zhi Eng 1001438448 14 Muhammad Syahizul Aidi Bin Berahim 1001438744 14 Ng Kuan Lim 1001436791 14 Phavanjeet Kaur A/P Avtar Singh 1001438581 14 Timothy Tay Kee Hock 1001438323 15 Nurul Hafizah Binti Nawawi 1001335439 15 Celia Wee Siew Pin 1001334730 15 Tharshini Devi A/P Krishna Moorthy 1001334526 15 Gary Poh Kwor Xiang 1001437556 16 Tan Ding Howe 1001437250 16 Steven Sia Chiew Weng 1001436640 16 Yeap Mei Hong 1001334459 16 Chew Zhi Hong 1001334698 17 Chin Zhi Ping 1001436623 17 Lai Ming Hui 1001334074 17 'Abdallah Suwaidan Salim Nasib Al-Quraini 1001436501 17
- 16. Responsibility of group members  Study group: to help each other to excel together in this course (EP204).  If ALL group members achieve more than 70% (14/20) in Test; 4/20 bonus marks will be added in their Midterm marks.  If ALL group members achieve more than 70% (14/20) in Midterm Test, 5/50 bonus marks will be added in Final Exam marks.  To cooperate as a team in group assignment and able to effectively plan, manage and solve the given tasks.
Editor's Notes
- #9: Remind for student: if they are sick (fever, etc.) during the exam (either internal or final exam), they are allowed to absent during the exam with a first sitting (100% counted) replacement but student MUST bring the MC not later than 3 days after the exam schedule.
- #12: Practice: Find friends who can help you success. Who can answer all the doubts. Students are encouraged to join PAL (Peer assisted learning) session (FREE tuition) – refer to lms/ respective HOD of your programmes Study smart: Find effective ways to study, to avoid repeating the same mistakes (especially for repeat students) Be calm and confidence: in the class, to practice and to answer the exams.