Lesson 5 Lewis Dot Structure of Molecular Covalent Compounds.pptx
Download as PPTX, PDF0 likes0 views
The Lewis dot structure (or Lewis structure) is a diagram that represents the valence electrons of atoms within a molecule. It helps visualize how atoms bond and share electrons to form molecules.
1 of 25
Download to read offline























Recommended
Lesson 5 Plan on How to Make Family Firmer and Gentler.pptx



Lesson 5 Plan on How to Make Family Firmer and Gentler.pptxMaryAnnLazarteBesar
╠²
Finding ways on how to make the family members firmer and gentler with each other
Lesson 5 in Earth and Life Science-Types of Stress and Fault.pptx



Lesson 5 in Earth and Life Science-Types of Stress and Fault.pptxMaryAnnLazarteBesar
╠²
Stress is the force applied to rocks in the Earth's crust, causing them to deform, bend, or breakLesson 8 in Earth and Life Science -Seafloor Spreading



Lesson 8 in Earth and Life Science -Seafloor SpreadingMaryAnnLazarteBesar
╠²
Seafloor Spreading is the process in which new oceanic crust forms at mid-ocean ridges and moves outward, pushing older crust away. This occurs due to the movement of tectonic plates and is a key mechanism of plate tectonicsLesson 6 in Erath and Life science- Layers of the Earth.



Lesson 6 in Erath and Life science- Layers of the Earth.MaryAnnLazarteBesar
╠²
The layers of the Earth are divided into four main parts, each with unique properties and compositions:atmosphere,biosphere, geosphere and hydrosphere
Lesson 7 in Earth and Life science Continental Drift



Lesson 7 in Earth and Life science Continental DriftMaryAnnLazarteBesar
╠²
Continental Drift is the theory that Earth's continents have moved over geological time and were once joined together in a single supercontinent called Pangaea. Proposed by Alfred Wegener in 1912, the theory suggests that the continents slowly drift across the Earth's surface due to movements in the Earth's mantle.Lesson 1 in Earth and Life Science - Weathering



Lesson 1 in Earth and Life Science - WeatheringMaryAnnLazarteBesar
╠²
Weathering is the natural process that breaks down rocks, soil, and minerals into smaller pieces over time due to exposure to the atmosphere, water, and living organismsLesson 5 Developmental Changes In Middle and Late Adolescence.pptx



Lesson 5 Developmental Changes In Middle and Late Adolescence.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 5Lesson 4 Developmental-Task-and-Challenges-of-Adolescence.pptx



Lesson 4 Developmental-Task-and-Challenges-of-Adolescence.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 4
Lesson 2 Unique Characteristics , Habits, and Experiences.pptx



Lesson 2 Unique Characteristics , Habits, and Experiences.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 2Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptx



Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 3Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Personal development Lesson 1
Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptx



Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 3Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Personal Development Lesson 1
Lesson 2 Astronomical Event before the advent of telescope.pptx



Lesson 2 Astronomical Event before the advent of telescope.pptxMaryAnnLazarteBesar
╠²
Quarter 4 Lesson in Physical ScienceLesson 1 How Greeks Knew that the Earth is Spherical.pptx



Lesson 1 How Greeks Knew that the Earth is Spherical.pptxMaryAnnLazarteBesar
╠²
Quarter 4 Lesson in Physical SciencePERDEV2- L2 Areas of Personal Devlopment.pptx



PERDEV2- L2 Areas of Personal Devlopment.pptxMaryAnnLazarteBesar
╠²
Third quarter lesson in Personal developmentFomulas of Common Chemical Substances.pptx



Fomulas of Common Chemical Substances.pptxMaryAnnLazarteBesar
╠²
Knowing the formulas of common chemical substances is highly significant for several reasons, ranging from practical applications in daily life to scientific and industrial advancements. The formula of a substance provides crucial information about its composition, structure, and behavior, which are important in many fieldsUsing Properties of Matter in Identifying Substances



Using Properties of Matter in Identifying SubstancesMaryAnnLazarteBesar
╠²
The properties of matter are crucial in identifying substances because they provide distinguishing characteristics that allow us to differentiate one substance from anotherComparing Consumer Products on the basis of their components for use, safety,...



Comparing Consumer Products on the basis of their components for use, safety,...MaryAnnLazarteBesar
╠²
Consumer products are goods that are purchased by individuals or households for personal use, rather than for business purposes. These products satisfy the needs and wants of consumers and are typically sold through retail outlets, online stores, or direct sales.HISTORY OF UNIVERSE Lesson 1 in Earth and Life science



HISTORY OF UNIVERSE Lesson 1 in Earth and Life scienceMaryAnnLazarteBesar
╠²
The widely accepted scientific explanation for the origin of the universe is the Big Bang Theory. This theory describes how the universe began as an incredibly dense and hot singularity and has been expanding and evolving over time
TheRuminantDigestiveSystem Lesson in Life Science for Grade 11



TheRuminantDigestiveSystem Lesson in Life Science for Grade 11MaryAnnLazarteBesar
╠²
The ruminant digestive system is a specialized system found in animals like cows, sheep, goats, deer, and buffalo. It is adapted for digesting plant-based diets, particularly fibrous materials like grass, which are difficult to break down. The system allows these animals to efficiently extract nutrients from cellulose-rich plant matter.Plant_Structures.ppt on Life Science for Grade 11



Plant_Structures.ppt on Life Science for Grade 11MaryAnnLazarteBesar
╠²
Plant structure refers to the physical organization and arrangement of different tissues, organs, and systems that make up a plant. It includes the study of both the macroscopic (visible) and microscopic (cellular and tissue-level) components of plantsPhysical science Lesson 3 Counting Atoms.pptx



Physical science Lesson 3 Counting Atoms.pptxMaryAnnLazarteBesar
╠²
ChatGPT
Explore GPTs
Today
Counting Atoms Explained
Previous 30 Days
GMO vs Selective Breeding
Cold Food for Sore Throat
Peanut Cough Trigger
Typhoon Track Explanation
Planaria Multiple-Choice Test
Vascular System vs Stem
Why Igneous Rocks Work
Magmatism Multiple Choice Test
Harry Hess Theory
Wegener's Continental Drift Theory
Strata vs Stratum
What is Strata
Nongreen Leaves Photosynthesis
Secondhand Smoke Risks
Ocean Basin Quiz
Plant Organ Systems Quiz
Capillarity Explained
Epidermal Tissue Structures
Meaning of to wit
November
Opening and Closing Oceans
Amoeba Digestive System Questions
Dental Pad Explained
Essay Writing Outline Tips
What are Protozoans
Ruminants and Cloaca Comparison
Ocean Floor vs Ocean Basin
October
Seafloor Spreading Questions
Upgrade plan
More access to the best models
ChatGPT
Share
User
You said:
how is counting of atoms done?
ChatGPT said:
ChatGPT
Counting atoms involves determining the number of individual atoms present in a molecule, compound, or mixture. This process can be done in various contexts, such as in chemical formulas, reactions, or physical measurements. Here's how it can be approached:
A chemical formula indicates the number and type of atoms in a compound. For example,
H
2
O
H
2
ŌĆŗ
O represents water, with two hydrogen atoms and one oxygen atom.
Subscripts indicate the number of atoms of an element directly preceding it. For example, in
C
6
H
12
O
6
C
6
ŌĆŗ
H
12
ŌĆŗ
O
6
ŌĆŗ
(glucose), there are:
6 Carbon atoms
12 Hydrogen atoms
6 Oxygen atomsPhysical science Lesson 2 Synthesis of New Elements.pptx



Physical science Lesson 2 Synthesis of New Elements.pptxMaryAnnLazarteBesar
╠²
New elements are synthesized by creating atomic nuclei that are heavier than those naturally found on Earth. This process involves nuclear reactions in which smaller atomic nuclei combine to form a larger, heavier nucleus. The creation of new elements typically occurs in specialized facilities like particle accelerators or nuclear reactors.Physical science Lesson 1 Formation of Light and Heavy Elements.pptx



Physical science Lesson 1 Formation of Light and Heavy Elements.pptxMaryAnnLazarteBesar
╠²
The synthesis of light and heavy elements occurs through various nuclear processes in stars and other cosmic environments. Physical Science Lesson 3 Counting Atoms.



Physical Science Lesson 3 Counting Atoms.MaryAnnLazarteBesar
╠²
Counting atoms involves determining the number of individual atoms present in a molecule, compound, or mixture. This process can be done in various contexts, such as in chemical formulas, reactions, or physical measurementsLesson 2 Synthesis of New Element- s.pptx



Lesson 2 Synthesis of New Element- s.pptxMaryAnnLazarteBesar
╠²
New elements are synthesized by bombarding nuclei with high-energy particles, which can cause them to change into a different, stable nucleus or break apart. As of 2013, the periodic table contains 114 confirmed elements up to element 118, the names of which were approved by IUPAC.Lesson 1 Formation of Light and Heavy Elements.pptx



Lesson 1 Formation of Light and Heavy Elements.pptxMaryAnnLazarteBesar
╠²
Some of the heavier elements in the periodic table are created when pairs of neutron stars collide cataclysmically and explode, researchers have shown for the first time. Light elements like hydrogen and helium formed during the big bang, and those up to iron are made by fusion in the cores of stars.Improving the Perturbation-Based Explanation of Deepfake Detectors Through th...



Improving the Perturbation-Based Explanation of Deepfake Detectors Through th...VasileiosMezaris
╠²
Presentation of our paper, "Improving the Perturbation-Based Explanation of Deepfake Detectors Through the Use of Adversarially-Generated Samples", by K. Tsigos, E. Apostolidis and V. Mezaris. Presented at the AI4MFDD Workshop of the IEEE/CVF Winter Conference on Applications of Computer Vision (WACV 2025), Tucson, AZ, USA, Feb. 2025. Preprint and software available at http://arxiv.org/abs/2502.03957 https://github.com/IDT-ITI/Adv-XAI-DeepfakesDetection of ferrihydrite in Martian red dust records ancient cold and wet co...



Detection of ferrihydrite in Martian red dust records ancient cold and wet co...S├®rgio Sacani
╠²
Iron oxide-hydroxide minerals in Martian dust provide crucial insights into
MarsŌĆÖ past climate and habitability. Previous studies attributed MarsŌĆÖ red color
to anhydrous hematite formed through recent weathering. Here, we show that
poorly crystalline ferrihydrite (Fe5O8H ┬Ę nH2O) is the dominant iron oxidebearing phase in Martian dust, based on combined analyses of orbital, in-situ,
and laboratory visible near-infrared spectra. Spectroscopic analyses indicate
that a hyperfine mixture of ferrihydrite, basalt and sulfate best matches Martian dust observations. Through laboratory experiments and kinetic calculations, we demonstrate that ferrihydrite remains stable under present-day
Martian conditions, preserving its poorly crystalline structure. The persistence
of ferrihydrite suggests it formed during a cold, wet period on early Mars
under oxidative conditions, followed by a transition to the current hyper-arid
environment. This finding challenges previous models of continuous dry oxidation and indicates that ancient Mars experienced aqueous alteration before
transitioning to its current desert state.More Related Content
More from MaryAnnLazarteBesar (20)
Lesson 2 Unique Characteristics , Habits, and Experiences.pptx



Lesson 2 Unique Characteristics , Habits, and Experiences.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 2Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptx



Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 3Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Personal development Lesson 1
Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptx



Lesson 3 Thoughts Feelings and Behaviors in Actual Life Situations.pptxMaryAnnLazarteBesar
╠²
Personal Development Lesson 3Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Personal Development Lesson 1
Lesson 2 Astronomical Event before the advent of telescope.pptx



Lesson 2 Astronomical Event before the advent of telescope.pptxMaryAnnLazarteBesar
╠²
Quarter 4 Lesson in Physical ScienceLesson 1 How Greeks Knew that the Earth is Spherical.pptx



Lesson 1 How Greeks Knew that the Earth is Spherical.pptxMaryAnnLazarteBesar
╠²
Quarter 4 Lesson in Physical SciencePERDEV2- L2 Areas of Personal Devlopment.pptx



PERDEV2- L2 Areas of Personal Devlopment.pptxMaryAnnLazarteBesar
╠²
Third quarter lesson in Personal developmentFomulas of Common Chemical Substances.pptx



Fomulas of Common Chemical Substances.pptxMaryAnnLazarteBesar
╠²
Knowing the formulas of common chemical substances is highly significant for several reasons, ranging from practical applications in daily life to scientific and industrial advancements. The formula of a substance provides crucial information about its composition, structure, and behavior, which are important in many fieldsUsing Properties of Matter in Identifying Substances



Using Properties of Matter in Identifying SubstancesMaryAnnLazarteBesar
╠²
The properties of matter are crucial in identifying substances because they provide distinguishing characteristics that allow us to differentiate one substance from anotherComparing Consumer Products on the basis of their components for use, safety,...



Comparing Consumer Products on the basis of their components for use, safety,...MaryAnnLazarteBesar
╠²
Consumer products are goods that are purchased by individuals or households for personal use, rather than for business purposes. These products satisfy the needs and wants of consumers and are typically sold through retail outlets, online stores, or direct sales.HISTORY OF UNIVERSE Lesson 1 in Earth and Life science



HISTORY OF UNIVERSE Lesson 1 in Earth and Life scienceMaryAnnLazarteBesar
╠²
The widely accepted scientific explanation for the origin of the universe is the Big Bang Theory. This theory describes how the universe began as an incredibly dense and hot singularity and has been expanding and evolving over time
TheRuminantDigestiveSystem Lesson in Life Science for Grade 11



TheRuminantDigestiveSystem Lesson in Life Science for Grade 11MaryAnnLazarteBesar
╠²
The ruminant digestive system is a specialized system found in animals like cows, sheep, goats, deer, and buffalo. It is adapted for digesting plant-based diets, particularly fibrous materials like grass, which are difficult to break down. The system allows these animals to efficiently extract nutrients from cellulose-rich plant matter.Plant_Structures.ppt on Life Science for Grade 11



Plant_Structures.ppt on Life Science for Grade 11MaryAnnLazarteBesar
╠²
Plant structure refers to the physical organization and arrangement of different tissues, organs, and systems that make up a plant. It includes the study of both the macroscopic (visible) and microscopic (cellular and tissue-level) components of plantsPhysical science Lesson 3 Counting Atoms.pptx



Physical science Lesson 3 Counting Atoms.pptxMaryAnnLazarteBesar
╠²
ChatGPT
Explore GPTs
Today
Counting Atoms Explained
Previous 30 Days
GMO vs Selective Breeding
Cold Food for Sore Throat
Peanut Cough Trigger
Typhoon Track Explanation
Planaria Multiple-Choice Test
Vascular System vs Stem
Why Igneous Rocks Work
Magmatism Multiple Choice Test
Harry Hess Theory
Wegener's Continental Drift Theory
Strata vs Stratum
What is Strata
Nongreen Leaves Photosynthesis
Secondhand Smoke Risks
Ocean Basin Quiz
Plant Organ Systems Quiz
Capillarity Explained
Epidermal Tissue Structures
Meaning of to wit
November
Opening and Closing Oceans
Amoeba Digestive System Questions
Dental Pad Explained
Essay Writing Outline Tips
What are Protozoans
Ruminants and Cloaca Comparison
Ocean Floor vs Ocean Basin
October
Seafloor Spreading Questions
Upgrade plan
More access to the best models
ChatGPT
Share
User
You said:
how is counting of atoms done?
ChatGPT said:
ChatGPT
Counting atoms involves determining the number of individual atoms present in a molecule, compound, or mixture. This process can be done in various contexts, such as in chemical formulas, reactions, or physical measurements. Here's how it can be approached:
A chemical formula indicates the number and type of atoms in a compound. For example,
H
2
O
H
2
ŌĆŗ
O represents water, with two hydrogen atoms and one oxygen atom.
Subscripts indicate the number of atoms of an element directly preceding it. For example, in
C
6
H
12
O
6
C
6
ŌĆŗ
H
12
ŌĆŗ
O
6
ŌĆŗ
(glucose), there are:
6 Carbon atoms
12 Hydrogen atoms
6 Oxygen atomsPhysical science Lesson 2 Synthesis of New Elements.pptx



Physical science Lesson 2 Synthesis of New Elements.pptxMaryAnnLazarteBesar
╠²
New elements are synthesized by creating atomic nuclei that are heavier than those naturally found on Earth. This process involves nuclear reactions in which smaller atomic nuclei combine to form a larger, heavier nucleus. The creation of new elements typically occurs in specialized facilities like particle accelerators or nuclear reactors.Physical science Lesson 1 Formation of Light and Heavy Elements.pptx



Physical science Lesson 1 Formation of Light and Heavy Elements.pptxMaryAnnLazarteBesar
╠²
The synthesis of light and heavy elements occurs through various nuclear processes in stars and other cosmic environments. Physical Science Lesson 3 Counting Atoms.



Physical Science Lesson 3 Counting Atoms.MaryAnnLazarteBesar
╠²
Counting atoms involves determining the number of individual atoms present in a molecule, compound, or mixture. This process can be done in various contexts, such as in chemical formulas, reactions, or physical measurementsLesson 2 Synthesis of New Element- s.pptx



Lesson 2 Synthesis of New Element- s.pptxMaryAnnLazarteBesar
╠²
New elements are synthesized by bombarding nuclei with high-energy particles, which can cause them to change into a different, stable nucleus or break apart. As of 2013, the periodic table contains 114 confirmed elements up to element 118, the names of which were approved by IUPAC.Lesson 1 Formation of Light and Heavy Elements.pptx



Lesson 1 Formation of Light and Heavy Elements.pptxMaryAnnLazarteBesar
╠²
Some of the heavier elements in the periodic table are created when pairs of neutron stars collide cataclysmically and explode, researchers have shown for the first time. Light elements like hydrogen and helium formed during the big bang, and those up to iron are made by fusion in the cores of stars.Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can Make A Person Accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...



Lesson 1 Knowing Oneself Can MAke a Person accept His/Her Strenghts and Limit...MaryAnnLazarteBesar
╠²
Comparing Consumer Products on the basis of their components for use, safety,...



Comparing Consumer Products on the basis of their components for use, safety,...MaryAnnLazarteBesar
╠²
Recently uploaded (20)
Improving the Perturbation-Based Explanation of Deepfake Detectors Through th...



Improving the Perturbation-Based Explanation of Deepfake Detectors Through th...VasileiosMezaris
╠²
Presentation of our paper, "Improving the Perturbation-Based Explanation of Deepfake Detectors Through the Use of Adversarially-Generated Samples", by K. Tsigos, E. Apostolidis and V. Mezaris. Presented at the AI4MFDD Workshop of the IEEE/CVF Winter Conference on Applications of Computer Vision (WACV 2025), Tucson, AZ, USA, Feb. 2025. Preprint and software available at http://arxiv.org/abs/2502.03957 https://github.com/IDT-ITI/Adv-XAI-DeepfakesDetection of ferrihydrite in Martian red dust records ancient cold and wet co...



Detection of ferrihydrite in Martian red dust records ancient cold and wet co...S├®rgio Sacani
╠²
Iron oxide-hydroxide minerals in Martian dust provide crucial insights into
MarsŌĆÖ past climate and habitability. Previous studies attributed MarsŌĆÖ red color
to anhydrous hematite formed through recent weathering. Here, we show that
poorly crystalline ferrihydrite (Fe5O8H ┬Ę nH2O) is the dominant iron oxidebearing phase in Martian dust, based on combined analyses of orbital, in-situ,
and laboratory visible near-infrared spectra. Spectroscopic analyses indicate
that a hyperfine mixture of ferrihydrite, basalt and sulfate best matches Martian dust observations. Through laboratory experiments and kinetic calculations, we demonstrate that ferrihydrite remains stable under present-day
Martian conditions, preserving its poorly crystalline structure. The persistence
of ferrihydrite suggests it formed during a cold, wet period on early Mars
under oxidative conditions, followed by a transition to the current hyper-arid
environment. This finding challenges previous models of continuous dry oxidation and indicates that ancient Mars experienced aqueous alteration before
transitioning to its current desert state.2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...



2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...Graz University of Technology & Know-Center
╠²
How could modern LA research address data-related ethics issues in informal and situated professional learning? I will identify in this talk three relevant insights based on field studies around workplace LA interventions: Firstly, in informal and situated learning, data isnŌĆÖt just about the learners. Secondly, the affordances of manual and automatic data tracking for learning are very different, with manual tracking allowing a high degree of learner control over data. Thirdly, learning is not necessarily a shared goal in workplaces. These can be translated into seeing a potential for systems endowed with sufficient natural-language-processing capability (now seemingly at our fingertips with LLMs), and socio-technical design and scenario-based data collection analysis as design and research methods.Blotting techniques and types of blotting .pptx



Blotting techniques and types of blotting .pptxsakshibhongal26
╠²
Blotting techniques- types and advantages, disadvantages WORKING AND APPLICATION OF LC-MS/MS 2025



WORKING AND APPLICATION OF LC-MS/MS 2025PSG College of Technology
╠²
LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) is a powerful analytical tool for comparing innovator and biosimilar drugs. It ensures precise characterization, detecting structural variations, impurities, and post-translational modifications, ensuring biosimilar quality, efficacy, and regulatory compliance in pharmaceutical development.Scientific Pig Farming Manual for Pig Farmers



Scientific Pig Farming Manual for Pig FarmersDr. Subhrajit Das
╠²
Pig farming, pork farming, pig production or hog farming is the raising and breeding of domestic pigs as livestock, and is a branch of animal husbandry. Pigs are farmed principally for food (e.g. pork: bacon, ham, gammon) and skins.
Pigs are amenable to many different styles of farming: intensive commercial units, commercial free range enterprises, or extensive farming (being allowed to wander around a village, town or city, or tethered in a simple shelter or kept in a pen outside the owner's house). Historically, farm pigs were kept in small numbers and were closely associated with the residence of the owner, or in the same village or town.[1] They were valued as a source of meat and fat, and for their ability to convert inedible food into meat and manure, and were often fed household food waste when kept on a homestead.[2] Pigs have been farmed to dispose of municipal garbage on a large scale.[3]
All these forms of pig farm are in use today, though intensive farms are by far the most popular, due to their potential to raise a large amount of pigs in a very cost-efficient manner.[4] In developed nations, commercial farms house thousands of pigs in climate-controlled buildings.[5] Pigs are a popular form of livestock, with more than one billion pigs butchered each year worldwide, 100 million in the United States. The majority of pigs are used for human food, but also supply skin, fat and other materials for use in clothing, ingredients for processed foods,[6] cosmetics,[7] and medical use.[8]Pig farming has gained importance today. Pigs have inherited capacity to acclimatize with varying climatic conditions. Pigs cannot withstand high temperature climate.
Pigs are adjusted to varied rearing practices and consume different types of food (Omnivorous) to attain higher growth and meat production.
Pigs will attain 60-70kg body weight in 6-8months period.
Female pigs i.e., sows will come to heat at age of 8-9 months but avoid using male pigs (Boars) for breeding purpose until it attains one year of age.
Adult sows when bred during right time after attaining maturity will farrow 8-12 piglets in 112-118 days of gestation period (i.e., about 4 months of gestation). Feedefficiencyis to gain one Kg live weightfor every 2.75-3kg feed consumed (FCR: 1:2.75). There are many advantageous in pig rearing. Pork is available at a cheaper price with nutritious and highly palatable tasty meat of higher quality animal protein. Pig bones are used for producing bone meal and also used for purification of sugar in sugar industry.
The manure droppings and urine are good fertilizers which enhance the soil fertilityand improve grain production.
Pig hairs (Bristles) are used for making brushes and ropes, hooves are used for shirt button making and preparation of gum. Hence, pigs are called as ŌĆ£multi utility domestic animalsŌĆØ. Farmers can take up piggery farming and reduce their debt burden and improve their profits and livelihood.
Preparing Ultrasound Imaging Data for Artificial Intelligence Tasks: Anonymis...



Preparing Ultrasound Imaging Data for Artificial Intelligence Tasks: Anonymis...ThrombUS+ Project
╠²
At the BIOSTEC 2025 conference, Eleni Kaldoudi, ThrombUS+ project coordinator, presented our recent work entitled ŌĆ£Preparing Ultrasound Imaging Data for Artificial Intelligence Tasks: Anonymisation, Cropping, and TaggingŌĆØ. Eleni provided an overview of the application we developed to facilitate the preparation of ultrasound images, acquired via the ThrombUS+ clinical study A, for the purpose of developing AI models for automated detection of deep vein thrombosis.
About ThrombUS+:
Our interdisciplinary approach centers around creating a novel wearable diagnostic device utilizing autonomous, AI-driven DVT detection. This groundbreaking device incorporates wearable ultrasound hardware, impedance plethysmography, and light reflection rheography for early clot detection. Activity and physiological measurements will continuously assess DVT risk, supporting prevention through serious gaming. An intelligent decision support unit will provide real-time monitoring and alerts, with extended reality guiding users for optimal device utilization.
ThrombUS+ is designed for postoperative patients, those undergoing lengthy surgical procedures, cancer patients, bedridden individuals at home or in care units, and women during pregnancy and postpartum.Investigational New drug application process



Investigational New drug application processonepalyer4
╠²
This file basically contains information related to IND application process in order to get approval for clinical trials.B-FPGM: Lightweight Face Detection via Bayesian-Optimized Soft FPGM Pruning



B-FPGM: Lightweight Face Detection via Bayesian-Optimized Soft FPGM PruningVasileiosMezaris
╠²
Presentation of our paper, "B-FPGM: Lightweight Face Detection via Bayesian-Optimized Soft FPGM Pruning", by N. Kaparinos and V. Mezaris. Presented at the RWS Workshop of the IEEE/CVF Winter Conference on Applications of Computer Vision (WACV 2025), Tucson, AZ, USA, Feb. 2025. Preprint and software available at http://arxiv.org/abs/2501.16917 https://github.com/IDT-ITI/B-FPGMDigestive System - Digestion of carbohydrates, proteins and lipids.ppt



Digestive System - Digestion of carbohydrates, proteins and lipids.pptJamakala Obaiah
╠²
Useful to studentsCell Structure & Function | Cambridge IGCSE Biology



Cell Structure & Function | Cambridge IGCSE BiologyBlessing Ndazie
╠²
This IGCSE Biology presentation provides a detailed look at cell structure and function, covering the differences between animal and plant cells, the roles of organelles (nucleus, mitochondria, ribosomes, etc.), specialized cells, and levels of organization. Learn about diffusion, osmosis, and active transport in cells, with clear diagrams and explanations to support exam preparation. A must-have resource for Cambridge IGCSE students!AUTOSOMES , ALLOSOMES AND SEX RATIO IN HUMAN POPULATION



AUTOSOMES , ALLOSOMES AND SEX RATIO IN HUMAN POPULATIONNistarini College, Purulia (W.B) India
╠²
This presentation offers a bird's eye view of autosomes and sex chromosomes. It also explores the different kinds of diseases of humans due to autosomal and sex-linked inherited traits. The sex determination of plants has been explained. The ratio of sex in the human population along with cause and consequences has been explained here.Electrical Quantities and Circuits | IGCSE Physics



Electrical Quantities and Circuits | IGCSE PhysicsBlessing Ndazie
╠²
This extensive slide deck provides a detailed exploration of electrical quantities and circuits for IGCSE Physics. It covers key electrical quantities, including charge, current, voltage (potential difference), resistance, power, energy, electromotive force (EMF), and internal resistance. The presentation also explains series and parallel circuits, with in-depth discussions on OhmŌĆÖs Law, KirchhoffŌĆÖs Laws, electrical components, circuit calculations, and practical applications. Packed with illustrative diagrams, worked examples, and exam-style questions, this resource is ideal for IGCSE students, teachers, and independent learners preparing for exams.2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...



2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...Graz University of Technology & Know-Center
╠²
Lesson 5 Lewis Dot Structure of Molecular Covalent Compounds.pptx
- 1. Objective ŌĆóDraw the Lewis dot structure of molecular covalent compounds
- 2. ŌĆó Lewis Structure Assumptions ŌĆóOnly valence electrons are involved in bonding. ŌĆóAtoms in molecules need eight valance electrons (octet rule) except for hydrogen which needs two electrons (duet rule).
- 3. ŌĆóIn covalent compounds atoms share electrons to form bonds in order to achieve stable noble gas electron configurations. ŌĆóIn ionic compounds electrons are transferred from one atom to another to achieve stable noble gas electron configurations
- 4. . Types of bond based on the Number of Shared Electron Pairs Single bond = 2 electrons to each atom ŌĆóDouble bond = 4 electrons to each atom ŌĆóTriple Bond = 6 electrons to each atom ŌĆóQuadruple Bond = 8 electrons to each atom
- 5. ŌĆóQuadruple bonds only exist between transition metals, such as rhenium, molybdenum, chromium, and tungsten, due to the availability of d-orbitals that enable unique bonding interactions
- 6. ŌĆóRules for Drawing Lewis Structures ŌĆóStep 1: Count the total number of valance electrons. ŌĆóStep 2: Identify the central atom (the first atom written unless that atom is hydrogen). ŌĆóPlace all terminal atoms around that atom. ŌĆóHydrogen atoms NEVER have more than one bond.
- 7. ŌĆóStep 3: Complete the octet for all atoms in the Lewis structure with lone pairs of electrons (except hydrogen). ŌĆóStep 4: Check your structure by counting the number of valance electrons used (they will match step 1 if the structure is correct). ŌĆó If your valance electrons donŌĆÖt match you will need to tweak your structure.
- 8. ŌĆóExample 1: CH4 ŌĆóStep 1 ŌĆó Count the number of valence electrons ŌĆóC = 4 valence electrons ŌĆóH = 1 valence electron ŌĆóStep 2 ŌĆóIdentify the central atom. This is usually the first atom written. Exception: Hydrogen. If that is the first atom written, then use the second atom. ŌĆóExample: C H 4 ŌĆóCentral atom: C
- 9. ŌĆóStep 3: Complete the octet ŌĆóDraw each component as a (Lewis) dot diagram.
- 10. ŌĆóStep 4: Check your structure ŌĆóCheck your structure by counting the number of valance electrons used ŌĆóRemember:Hydrogen is the exception to the octet rule. It only needs 2 electrons to be happy. ŌĆóMake sure that each atom in your new Lewis dot structure ŌĆ£feelsŌĆØ like it has eight electrons around it. Remember: One bond ŌĆ£feelsŌĆØ like two electrons to EACH element
- 11. ŌĆó If here are atoms whose octet rules are not satisfied, you may need to increase the number of bonds between atoms. 2 2 2 2 8
- 12. ŌĆóExample 2: Water (HŌééO) 1.Valence electrons: 1.Oxygen: 6, Hydrogen: 1 each ŌåÆ Total = 8 2.Central atom: Oxygen (less electronegative than hydrogen) 3.Single bonds: Connect H to O with single bonds 4.Distribute remaining electrons: Place 4 more electrons as lone pairs on oxygen
- 13. Final structure:
- 14. ŌĆóExample 3: Carbon Dioxide (COŌéé) 1.Valence electrons: 1.Carbon: 4, Oxygen: 6 each ŌåÆ Total = 16 2.Central atom: Carbon (least electronegative) 3.Single bonds: Connect C to each O with single bonds 4.Distribute electrons: Each oxygen gets 6 remaining electrons (3 lone pairs), but carbon has only 4 electrons. To satisfy the octet rule, change single bonds to double bonds.
- 15. ŌĆóLewis Structures of CO2 (carbon dioxide)
- 16. Final structure:
- 17. ŌĆóLewis Structures CO (carbon monoxide)
- 18. ŌĆóŌĆ£ ExceptionsŌĆØ to the Octet ŌĆóIf you only have four or six valance electrons initially you canŌĆÖt possibly fill the octet rule (usually BeH2 or BH3 ).(Incomplete Octet) ŌĆó Just place hydrogens around central atoms and call it done. ŌĆóExceptions to the Octet Rule (That are not H) ŌĆóThere are two other exceptions to the Octet Rule (that are not Hydrogen) (Expanded Octet) ŌĆóExamples: ŌĆóPF5 ŌĆóSF6 ŌĆóExceptions usually involve F
- 19. PF5
- 20. SF6
- 21. Diatomic Molecules In nature, the following elements are always found in a paired molecule. They are never found solo. I2 Br2 Cl2 F2 O2 N2 H2 I Bring Clay For Our New House
- 22. ŌĆóSummary: ŌĆóSteps to Draw a Lewis Structure: 1.Count valence electrons 2. Determine the central atom The first element written and the least electronegative atom(except hydrogen) is usually the central atom. 3. Connect atoms with single bonds Draw single bonds (each bond = 2 electrons) between atoms.
- 23. 4. Distribute remaining electrons Complete octet (8 electrons) for outer atoms first, then for the central atom. 5. Check octet rule and adjust if needed If the central atom lacks an octet, form double or triple bonds. 6. Check the total number of valence electrons Make sure the number of electrons used matches the total count.
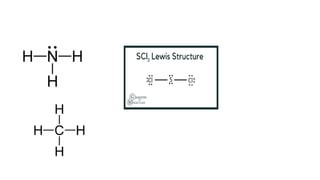
- 24. ŌĆóDraw the Lewis Dot Structure of the following compounds ŌĆó1. Ammonia (NHŌéā) ŌĆó2. Methane (CHŌéä) ŌĆó3. Sulfur dichloride (SCl2)






