Lesson plan on fire works!
- 1. CHEMISTRY OF FIRE WORKS Name of the teacher: Nithya S Nair Std: VIII Name of the School: GHSS Pallimon Strength: 42 Subject: Chemistry Duration: 45 min Unit: Changes Date: 12th July 2014 Topic: Chemistry of fire works CURRICULAR STATEMENT Through experiment, observation and discussion pupil understatnds about fire work CONTENT ANALYSIS Term: Fire works Facts:  Gun powder is the mixturemade by mixing potassium diamide, powdered sulphite and carbon powder  Burning of gun powder is a fast process and a lots of gases are formed CONCEPT Chemicals added with the gun powder to give beautiful colours to the flame. Salts of different metals ae added for this purpose Learning outcome: Pupil enable to develop  Factual knowledge on th topic, the chemistry of fire works a) Facts and terms mentioned in the content analysis b) Recognising different concepts related to fire works  Conceptual knowledge about the chemistry of fire works a) Recalling changes b) Recognising diffrent concepts related to fire works  Procedural knowledge about the chemistry of fire works a) Executing practical application about fire works
- 2. ÔÇ∑ Meta cognitive knowledge about the chemistry of fire works a) Executing practical works as per instructions ÔÇ∑ Positive attitudes towards the chemistry of fire works ÔÇ∑ Different process skills like a) Observation of different fire works b) Communicating through presenting group works Pre-requisites: Pupil know about chemical changes Teaching-learning resources: ∫›∫›fl£ presentation Class room interaction procedure: Process /Activity Actual response Did you see fire works? Do you like to watch fire works? Did you think how the flames are getting different colours? Can we learn about the secret behind the fire works? Yes Yes Yes yes ACTIVITY 1 After giving a short introduction about chemistry of fire wroks, teacher shows power point presentation http://www.slideshare.net/nithyasnair/chemistry-of-fire-works ACTIVITY 2 After the presentation teacher shows a video which shows fire works http://youtu.be/CNjggrxUQ78
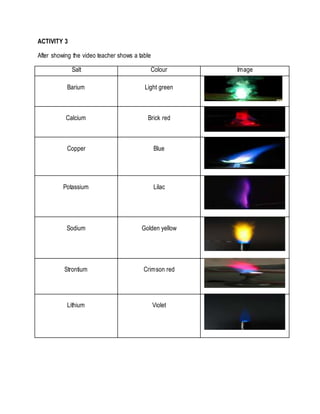
- 3. ACTIVITY 3 After showing the video teacher shows a table Salt Colour Image Barium Light green Calcium Brick red Copper Blue Potassium Lilac Sodium Golden yellow Strontium Crimson red Lithium Violet
- 4. Review Questions: 1. What are the chemicals used to make a gun powder ? 2. Find out the colour of the flame if we add a) Lithium b) Strontium c) Potassium 3. How varoius colours are given to the fire works? Follow up Acitivity Prepare a short note about the chemistry of fire works Self reflection This topic was done in the IT labby presenting slides about the chemistry of fire works. Pupils enjoyed this portionand I showed a small video about the fire works. By showing different pictures of fire worksI catogorised the salts and colour to the flame. So it was easy for me to convince the topic through slide presentation ***THE END**