preparation of calcium Titanate (CTO)
- 1. Synthesis of Calcium Titanate (CTO) By Harshal Chaudhari M.Sc. Part 2 17PHY203
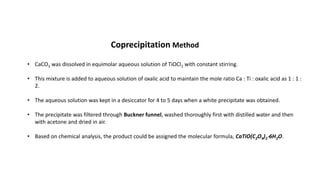
- 2. âĒ CaCO3 was dissolved in equimolar aqueous solution of TiOCl2 with constant stirring. âĒ This mixture is added to aqueous solution of oxalic acid to maintain the mole ratio Ca : Ti : oxalic acid as 1 : 1 : 2. âĒ The aqueous solution was kept in a desiccator for 4 to 5 days when a white precipitate was obtained. âĒ The precipitate was filtered through Buckner funnel, washed thoroughly first with distilled water and then with acetone and dried in air. âĒ Based on chemical analysis, the product could be assigned the molecular formula, CaTiO(C2O4)2â 6H2O. Coprecipitation Method
- 3. Heat CaTiO(C2O4)2â 6H2O in microwave/SiC furnace at 500â°C to 700 â° C for 1 hour. CaTiO(C2O4)2â 6H2O decomposes in 5 steps, âĒ CaTiO(C2O4) 2â 6H2O â CaTiO(C2O4)2 + 6H2O âĒ 2CaTiO(C2O4)2 â Ca2Ti2O5CO3(CO)2 + 3CO2 + 2CO âĒ Ca2Ti2O5CO3(CO)2 â Ca2Ti2O5CO3(CO) + CO âĒ Ca2Ti2O5CO3(CO) â CaTi2O5CO3 + CO âĒ Ca2Ti2O5(CO3)2 â 2CaTiO3 + CO2
- 4. Hydrothermal Reaction Precursor TiCl4, CaCl22H2O and KOH. In separate beakers TiCl4 and CaCl22H2O add slowly to de-ionized water under vigorous stirring, forming Ti4+ and Ca2+. Then, mix two solutions containing Ti4+ and Ca2+ ions to and add to 50 ml of the KOH solution (6 M) to bring pH = 14. The mixture then loaded into a 110 ml Polytetrafluoroethylene autoclave reaching 90% of its volume. Seal the autoclave and place into a microwave and heat to 140C. Heat for required time (depending upon the desired product) under pressure of 2.5 bar. Afterwards the autoclave is allowed to cool at room temperature then the solid is washed with water till the pH is neutralized. Then the power(CaTiO3) is kept for drying at 80â°C for 12 hours.
- 5. Mass calculation (units in g/mol) Solid State reaction CaO + TiO â CaTiO3 56.07 + 76.86 â 135.94 For 10g of CaTiO3 56.07/13.59 of CaO + 76.86/13.59 of TiO â 10g of CaTiO3 CaO = 4.12g TiO = 5.66g Co Precipitation method CaCO3 + TiOCl2 â CaTiO3 + C+4 + O+2 + Cl2 100.09 + 134.77 â 135.94 100.09/13.59 + 134.77/13.59 â 10 CaO = 7.36g ; TiO = 9.92g Hydrothermal reaction TiCl2 + CaCl22H20 â CaTiO3 + 2H2O + 2Cl2 118.77 + 147.01 â 135.94 118.77/13.59 + 147.01/13.59 â 10 CaO = 8.74g ; TiO = 10.82g
- 6. References B M PATIL, R S SRINIVASA and S R DHARWADKAR; âSynthesis of CaTiO3 from calcium titanyl oxalate hexahydrate (CTO) as precursor employing microwave heating techniqueâ; Bull. Mater. Sci., 2007, 30, 225â229. Mario L. Moreira a, Elaine C. Paris a, Gabriela S. do Nascimento a, Valeria M. Longo e, Julio R. Sambrano b, Valmor R. Mastelaro c, Maria I.B. Bernardi c, Juan AndreÂīs d, JoseÂī A. Varela e, Elson Longo e; âStructural and optical properties of CaTiO3 perovskite-based materials obtained by microwave-assisted hydrothermal synthesis: An experimental and theoretical insightâ; Acta Materialia 57 2009