MBTH FC-PDAB Reagents
0 likes755 views
This document describes several reagents used for drug analysis: MBTH, FC, PDAB, and 2,6-dichloroquinone chlorimide. MBTH reacts with phenols, amines, aldehydes and other compounds to form colored complexes. FC (Folin-Ciocalteu) reagent contains heteropoly acids and reduces to a blue color with reducing agents. PDAB (para-dimethylaminobenzaldehyde) forms azomethines with amines. 2,6-dichloroquinone chlorimide reacts with unsubstituted para-phenols to form a blue colored product. Examples of drug analyses using each reagent are provided.
1 of 17











Recommended
Pharmaceutical reagents, PDAB, FC, MBTH



Pharmaceutical reagents, PDAB, FC, MBTHDivya Naidu
Ěý
This document discusses various reagents used in pharmaceutical analysis including PDAB, Folin Ciocalteau, and MBTH reagents. It provides details on the principles, mechanisms, procedures, examples, and applications of each reagent. PDAB is used to detect primary amine groups via a colorimetric reaction. Folin Ciocalteau is used to detect phenols and reduces tungstate-molybdate to form a blue complex. MBTH forms colored complexes with aldehydes, phenols, and amines through oxidative coupling and can be used to analyze samples containing these functional groups. The document concludes that optimizing reagent volume and concentration allows these reagents to be successfully used to quantify drugs in pharmaceutical formulations.Principle and Applications Of MBTH, NQS, FC and BM Reagents



Principle and Applications Of MBTH, NQS, FC and BM ReagentsLakshmi Kalyani
Ěý
This document discusses MBTH, FC, and BM reagents which are used for qualitative and quantitative analysis of pharmaceuticals. It provides background on each reagent, including their chemical structure and properties. The principles and mechanisms of how each reagent reacts with different functional groups like phenols, amines, and aldehydes are described. Examples of using each reagent to estimate drugs are provided, such as using MBTH to analyze acyclovir and FC to determine protein concentration. The document concludes that these reagents can successfully quantify and qualify drugs in formulations when used at optimized concentrations.Notes* for the subject 'Advanced Pharmaceutical Analysis'



Notes* for the subject 'Advanced Pharmaceutical Analysis'Sanathoiba Singha
Ěý
As per the syllabus prescribed by Rajiv Gandhi University of Health Sciences, Karnataka, for M. Pharm (Pharmaceutical Analysis) 1st semester.
*not all topics have been included in this collection of notes.Principles & procedures involved in usage of reagents



Principles & procedures involved in usage of reagentskapil Patel
Ěý
The document discusses various reagents used in pharmaceutical analysis. It begins by classifying reagents based on their reaction mechanisms such as oxidation, condensation, diazotization, and others. Several examples of reagents are provided for each classification including MBTH, Folin-Ciocalteu reagent, PDAB, and 2,4-DNP. Their chemical properties and applications in estimating specific drugs are described. The document concludes by providing an example estimation procedure using MBTH reagent.Pdac,ninhydrin,1,2 naphthoquinone reagents



Pdac,ninhydrin,1,2 naphthoquinone reagentsthota lakshmi bhavani
Ěý
The document discusses various reagents used in pharmaceutical analysis including their principles, procedures, reactions, and applications. It describes Para Dimethylamino Cinnamaldehyde (PDAC) reagent which is used to determine hydrazine. It reacts with primary amines to form colored Schiff bases. Ninhydrin reagent is used to estimate drugs containing free amine groups and gives a purple color. 1,2-Naphthoquinone-4-sulfonic acid (NQS) reagent is widely used for drugs containing aromatic amines, replacing its sulfonate group with the amine group to form a colored chromogen. Examples provided estimate drugs like ranitidine, lenalidomide, and ampbio analytical method validation usfda guidlines



bio analytical method validation usfda guidlineschandu chatla
Ěý
This document provides guidance from the US FDA on bioanalytical method validation. It discusses key principles such as method development, validation parameters for chromatographic and ligand binding assays, calibration curves, quality controls, selectivity, sensitivity, accuracy, precision, recovery, and stability. The guidance is intended to help sponsors validate analytical methods used in human and animal studies to quantify drug, metabolite, protein, and biomarker levels in biological matrices like blood, serum and tissue.QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...



QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...CARE COLLEGE OF PHARMACY
Ěý
This document describes methods for quantitatively determining preservatives, emulsifiers, and colouring materials found in foods and pharmaceuticals. It discusses using thin-layer chromatography to identify common preservatives like methyl paraben and propyl paraben, and extracting and identifying emulsifiers like polysorbate 80. Gas chromatography methods are provided for determining mono- and diglycerides. Finally, it outlines extracting and identifying food dyes from candy coatings using yarn and ammonia.Bioassay of Heparin Sodium



Bioassay of Heparin SodiumSathiyaThaarani
Ěý
The document summarizes the bioassay of heparin sodium. The bioassay involves comparing the concentration of a standard heparin preparation needed to prevent clotting of plasma to that of the test heparin solution. Prepared plasma is mixed with increasing dilutions of both the standard and test heparin solutions and the degree of clotting is observed after 1 hour and used to determine the potency of the test sample relative to the standard. Heparin is an anticoagulant drug extracted from animal tissues and used to treat and prevent blood clots and thrombosis. Its potency is typically measured using clotting-based biological assays.Validation parameters



Validation parametersSandhya Chintalacheruvu
Ěý
This document discusses the key aspects of analytical method validation including specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, robustness, and system suitability testing. It provides detailed descriptions and recommendations for establishing each validation characteristic to demonstrate that an analytical procedure is suitable for its intended use.Advanced pharmaceutical analysis



Advanced pharmaceutical analysisNeha Suresh
Ěý
This document discusses the classification and control of elemental impurities in pharmaceutical products. It divides elements into three classes based on their toxicity and likelihood of occurrence in drugs. Class 1 elements are highly toxic and should be evaluated across all potential sources. Class 2 elements are route-dependent toxins divided into 2A and 2B based on probability of occurrence. Class 3 elements have relatively low oral toxicity but may warrant consideration for inhalation and injectable routes. The document also describes establishing permitted daily exposure limits, applying a risk-based approach to control impurities, and maintaining an overall control strategy throughout a product's lifecycle.extraction of drug from biological matrix.pptx



extraction of drug from biological matrix.pptxanumalagundam sreekanth
Ěý
This document discusses extraction of drugs and metabolites from biological matrices. It covers topics like sample preparation techniques, types of samples, physicochemical properties of drugs, and methods of extraction. The key goals of sample preparation are to isolate analytes from interfering compounds, dissolve them in a suitable solvent, and pre-concentrate them at a level suitable for detection. Common extraction methods mentioned are liquid-liquid extraction, solid phase extraction, protein precipitation, solid phase microextraction, matrix solid phase dispersion, and supercritical fluid extraction.Study of Quality of Raw Materials and General methods of analysis of Raw mate...



Study of Quality of Raw Materials and General methods of analysis of Raw mate...Raghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains Study of Quality of Raw Materials and General methods of analysis of Raw materials used in cosmetic manufacture as per BSI
Presented by: P.PAVAN KALYAN (Department of pharmaceutical analysis).RIPER, anantapurBasics impurity profiling and degradent characterization[134]![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
Basics impurity profiling and degradent characterization[134]University Institute of Pharmaceutical Sciences
Ěý
University Institute of Pharmaceutical Sciences is a flag bearer of excellence in Pharmaceutical education and research in the country. Here is another initiative to make study material available to everyone worldwide. Based on the new PCI guidelines and syllabus here we have a presentation dealing with basics impurity profiling and degradent characterization.
Thank you for reading.
Hope it was of help to you.
UIPS,PU teamImpurity profiling and degradent characterization {presented by shameer m.pha...



Impurity profiling and degradent characterization {presented by shameer m.pha...ShameerAbid
Ěý
these slides discuss
Impurity profiling
Degradation characterization
Stability testing & Accelerated stability testing (ICH)
Evaluation of the test (shelf life)
analytical method development
ICH vs USP definition
methods for identification
method for the isolation of the impurity
factors affecting the degradation of formulation
What is degradation characterization
general protocol of degradation conditions used for drug substance and drug product
Degradation conditions
Stress testing
Container closure system
Diazotization titrtions



Diazotization titrtionsShwetha M
Ěý
Diazotization titrations involve the reaction of primary aromatic amines with sodium nitrite in acidic solution to form unstable diazonium salts. This reaction can be used for both qualitative and quantitative analysis of compounds containing amino groups. The endpoint is detected using an external indicator like starch-iodide paper, which detects excess nitrous acid after all the aromatic amine has reacted. Some common compounds that can be assayed via diazotization titration include dapsone, sulphamethoxazole, and benzocaine.Physical stability tablets & capsules



Physical stability tablets & capsulesShweta Singh
Ěý
Stability test of Tablets, Capsules And Granules in order to check their shelf life and quality control.Lipids (Part-1) || Food Analysis || Pharmaceutical Analysis Department || M.P...



Lipids (Part-1) || Food Analysis || Pharmaceutical Analysis Department || M.P...saimuniswetha1
Ěý
Hello everyone,
Today we discuss about Lipids (part-1) in Food Analysis subject in M.pharmacy(Pharmaceutical Analysis Department)... Qualification of Analytical Equipments



Qualification of Analytical EquipmentsDhawal_Raghuvanshi
Ěý
The document discusses the qualification of analytical equipment, specifically gas chromatography. It describes the four parts of qualification: design qualification, installation qualification, operational qualification, and performance qualification. It then provides examples of tests and acceptance criteria for various parameters of a gas chromatography system, including the inlet system, oven, and flame ionization detector. Tests include leak tests, repeatability, linearity, noise, drift and more. The goal is to verify that the GC system is installed and functioning properly.Diazotization TITRATION FOR PG NOTES VERY USEFUL 



Diazotization TITRATION FOR PG NOTES VERY USEFUL prakash64742
Ěý
The document discusses the principles and applications of diazotization titration. Diazotization involves the reaction of an aromatic primary amine with sodium nitrite in acidic medium to form a diazonium salt. The titration endpoint is determined using an indicator reaction that detects excess nitrous acid. Common applications described include assays of benzocaine, dapsone, and isocarboxazid which involve diazotization and detection of the endpoint with starch-iodide paper. Diazotization titration can be used to analyze many sulfa drugs and other pharmaceuticals containing aromatic amine groups.Qualification of analytical instruments



Qualification of analytical instrumentsFaris ameen
Ěý
This document provides guidelines for qualifying analytical instruments including electronic balances, pH meters, and UV-Visible spectrophotometers. It discusses the various levels of qualification including: Level I which involves selecting instruments and suppliers; Level II which involves installation and releasing instruments for use; Level III which involves periodic checks; and Level IV which involves in-use checks. Specific guidelines are provided for qualifying balances, pH meters, and UV-Visible spectrophotometers, including recommended tolerance limits for various parameters, calibration procedures, and qualification frequencies.Notes for the subject 'Pharmaceutical Validation' 



Notes for the subject 'Pharmaceutical Validation' Sanathoiba Singha
Ěý
According to the syllabus prescribed by Rajiv Gandhi University of Health Sciences, Karnataka, for M. Pharm (Pharmaceutical Analysis) 1st semester.Evaluation of dosage forms



Evaluation of dosage formsShaik Sana
Ěý
The document outlines the evaluation of various solid, semi-solid, and liquid dosage forms. For solid dosage forms like tablets, important tests include general appearance, weight variation, content uniformity, hardness, friability, disintegration, and dissolution. These tests are conducted to ensure quality, consistency in drug content between tablets, and that the drug will release and dissolve properly. Similar tests are described for other dosage forms like capsules, granules, powders, ointments, creams, suppositories, syrups, suspensions, emulsions, and novel delivery systems. The goal of evaluation is to quantify properties and assess interactions that impact drug stability and bioavailability.General considerations and method development in ce,



General considerations and method development in ce,ChowdaryPavani
Ěý
This document provides an overview of capillary electrophoresis (CE). It defines CE, describes its principle and instrumentation. CE involves separating components of a sample based on their differential rate of migration in an applied electric field. Key points covered include electrophoretic mobility, electroosmotic flow, sample introduction techniques, and common applications such as protein, DNA and pharmaceutical analysis. CE provides high resolution separations due to its small capillary diameter and long separation length.Residual solvent 



Residual solvent Saptarshi Das
Ěý
This document summarizes the determination of residual solvents in pharmaceuticals by gas chromatography according to ICH guidelines. Residual solvents are organic chemicals used in manufacturing that are not fully removed and may pose health risks. They are classified into Class 1, 2, or 3 based on toxicity, with Class 1 solvents prohibited. Gas chromatography with headspace injection and flame ionization detection is the primary analytical method. It involves using capillary columns, temperature programs, and identifies solvents by comparing retention times to standards. The document outlines accepted chromatographic conditions and procedures A, B, and C described in pharmacopeias.IMPURITIES AND STABILITY STUDIES



IMPURITIES AND STABILITY STUDIESprakash64742
Ěý
This document summarizes a seminar presentation on impurities and stability studies. It defines impurities as any component of a drug substance that is not the defined chemical entity. Impurities are classified as organic, inorganic, or residual solvents. Organic impurities can arise from manufacturing processes or storage and include starting materials, byproducts, and degradation products. Inorganic impurities result from manufacturing and include reagents, metals, and salts. Residual solvents are volatile organic chemicals used in drug substance synthesis. The document also discusses ICH guidelines for qualifying impurities based on safety testing and provides a decision tree for conducting safety studies of drug substances.IMPURITY PROFILING (SOURCES OF IMPURITIES)



IMPURITY PROFILING (SOURCES OF IMPURITIES)N Anusha
Ěý
The description, characterization and quantitation of identified and unidentified impurities present in the drug substances is known as impurity profile.
IMPURITIES in pharmaceuticals are unwanted chemicals, that even in small amounts may influence the efficacy and safety of the pharmaceutical products.
Degradation and Degradant Characterization



Degradation and Degradant CharacterizationGagan Deep
Ěý
This document discusses degradation, degradants, and degradant characterization. It defines degradation as changes in a substance's properties due to environmental factors or formulation interactions. Degradants are products formed from degradation that can reduce potency or induce toxicity. Degradant characterization involves profiling degradants using analytical techniques like LC-MS, GC-MS, and LC-NMR to separate and identify degradants. Factors like temperature, moisture, light exposure, pH changes, and microbes can induce degradation.Forced Degradation Study 



Forced Degradation Study Sumant Baukhandi
Ěý
This document outlines stability testing recommendations for different drug formulations, including whether acid-base, oxidative, photo-stability, thermal, or thermal-humidity testing is necessary or optional. It also describes how forced degradation studies are used to evaluate drug substance and product stability in various conditions, identify possible degradation pathways and products, and facilitate improvements to manufacturing and formulations.Unit 3 furan & thiophene



Unit 3 furan & thiopheneSowmiya Perinbaraj
Ěý
1) Furan and thiophene are 5-membered heterocyclic compounds containing oxygen and sulfur respectively. They are prepared via various synthetic routes and undergo electrophilic substitution and addition reactions.
2) Furan and thiophene have several medicinal uses. Furan derivatives like furosemide and ranitidine are used as diuretics and antiulcer drugs. Thiophene derivatives are used as antibiotics, antihistamines, anticonvulsants and anti-inflammatory drugs.
3) The document discusses the structures, properties, synthesis and reactions of furan and thiophene. It also outlines some of their common medicinal applications.Phenols and amines



Phenols and aminesOksana Kamenetska
Ěý
Lecture "Phenols and amines" on Pharmaceutical Chemistry is devoted by synthesis, physico-chemical properties and analysis of medicinal substances, which are derivatives of phenols and aromatic amines.Also is included examples of tests "KROK-2".More Related Content
What's hot (20)
Validation parameters



Validation parametersSandhya Chintalacheruvu
Ěý
This document discusses the key aspects of analytical method validation including specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, robustness, and system suitability testing. It provides detailed descriptions and recommendations for establishing each validation characteristic to demonstrate that an analytical procedure is suitable for its intended use.Advanced pharmaceutical analysis



Advanced pharmaceutical analysisNeha Suresh
Ěý
This document discusses the classification and control of elemental impurities in pharmaceutical products. It divides elements into three classes based on their toxicity and likelihood of occurrence in drugs. Class 1 elements are highly toxic and should be evaluated across all potential sources. Class 2 elements are route-dependent toxins divided into 2A and 2B based on probability of occurrence. Class 3 elements have relatively low oral toxicity but may warrant consideration for inhalation and injectable routes. The document also describes establishing permitted daily exposure limits, applying a risk-based approach to control impurities, and maintaining an overall control strategy throughout a product's lifecycle.extraction of drug from biological matrix.pptx



extraction of drug from biological matrix.pptxanumalagundam sreekanth
Ěý
This document discusses extraction of drugs and metabolites from biological matrices. It covers topics like sample preparation techniques, types of samples, physicochemical properties of drugs, and methods of extraction. The key goals of sample preparation are to isolate analytes from interfering compounds, dissolve them in a suitable solvent, and pre-concentrate them at a level suitable for detection. Common extraction methods mentioned are liquid-liquid extraction, solid phase extraction, protein precipitation, solid phase microextraction, matrix solid phase dispersion, and supercritical fluid extraction.Study of Quality of Raw Materials and General methods of analysis of Raw mate...



Study of Quality of Raw Materials and General methods of analysis of Raw mate...Raghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains Study of Quality of Raw Materials and General methods of analysis of Raw materials used in cosmetic manufacture as per BSI
Presented by: P.PAVAN KALYAN (Department of pharmaceutical analysis).RIPER, anantapurBasics impurity profiling and degradent characterization[134]![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
Basics impurity profiling and degradent characterization[134]University Institute of Pharmaceutical Sciences
Ěý
University Institute of Pharmaceutical Sciences is a flag bearer of excellence in Pharmaceutical education and research in the country. Here is another initiative to make study material available to everyone worldwide. Based on the new PCI guidelines and syllabus here we have a presentation dealing with basics impurity profiling and degradent characterization.
Thank you for reading.
Hope it was of help to you.
UIPS,PU teamImpurity profiling and degradent characterization {presented by shameer m.pha...



Impurity profiling and degradent characterization {presented by shameer m.pha...ShameerAbid
Ěý
these slides discuss
Impurity profiling
Degradation characterization
Stability testing & Accelerated stability testing (ICH)
Evaluation of the test (shelf life)
analytical method development
ICH vs USP definition
methods for identification
method for the isolation of the impurity
factors affecting the degradation of formulation
What is degradation characterization
general protocol of degradation conditions used for drug substance and drug product
Degradation conditions
Stress testing
Container closure system
Diazotization titrtions



Diazotization titrtionsShwetha M
Ěý
Diazotization titrations involve the reaction of primary aromatic amines with sodium nitrite in acidic solution to form unstable diazonium salts. This reaction can be used for both qualitative and quantitative analysis of compounds containing amino groups. The endpoint is detected using an external indicator like starch-iodide paper, which detects excess nitrous acid after all the aromatic amine has reacted. Some common compounds that can be assayed via diazotization titration include dapsone, sulphamethoxazole, and benzocaine.Physical stability tablets & capsules



Physical stability tablets & capsulesShweta Singh
Ěý
Stability test of Tablets, Capsules And Granules in order to check their shelf life and quality control.Lipids (Part-1) || Food Analysis || Pharmaceutical Analysis Department || M.P...



Lipids (Part-1) || Food Analysis || Pharmaceutical Analysis Department || M.P...saimuniswetha1
Ěý
Hello everyone,
Today we discuss about Lipids (part-1) in Food Analysis subject in M.pharmacy(Pharmaceutical Analysis Department)... Qualification of Analytical Equipments



Qualification of Analytical EquipmentsDhawal_Raghuvanshi
Ěý
The document discusses the qualification of analytical equipment, specifically gas chromatography. It describes the four parts of qualification: design qualification, installation qualification, operational qualification, and performance qualification. It then provides examples of tests and acceptance criteria for various parameters of a gas chromatography system, including the inlet system, oven, and flame ionization detector. Tests include leak tests, repeatability, linearity, noise, drift and more. The goal is to verify that the GC system is installed and functioning properly.Diazotization TITRATION FOR PG NOTES VERY USEFUL 



Diazotization TITRATION FOR PG NOTES VERY USEFUL prakash64742
Ěý
The document discusses the principles and applications of diazotization titration. Diazotization involves the reaction of an aromatic primary amine with sodium nitrite in acidic medium to form a diazonium salt. The titration endpoint is determined using an indicator reaction that detects excess nitrous acid. Common applications described include assays of benzocaine, dapsone, and isocarboxazid which involve diazotization and detection of the endpoint with starch-iodide paper. Diazotization titration can be used to analyze many sulfa drugs and other pharmaceuticals containing aromatic amine groups.Qualification of analytical instruments



Qualification of analytical instrumentsFaris ameen
Ěý
This document provides guidelines for qualifying analytical instruments including electronic balances, pH meters, and UV-Visible spectrophotometers. It discusses the various levels of qualification including: Level I which involves selecting instruments and suppliers; Level II which involves installation and releasing instruments for use; Level III which involves periodic checks; and Level IV which involves in-use checks. Specific guidelines are provided for qualifying balances, pH meters, and UV-Visible spectrophotometers, including recommended tolerance limits for various parameters, calibration procedures, and qualification frequencies.Notes for the subject 'Pharmaceutical Validation' 



Notes for the subject 'Pharmaceutical Validation' Sanathoiba Singha
Ěý
According to the syllabus prescribed by Rajiv Gandhi University of Health Sciences, Karnataka, for M. Pharm (Pharmaceutical Analysis) 1st semester.Evaluation of dosage forms



Evaluation of dosage formsShaik Sana
Ěý
The document outlines the evaluation of various solid, semi-solid, and liquid dosage forms. For solid dosage forms like tablets, important tests include general appearance, weight variation, content uniformity, hardness, friability, disintegration, and dissolution. These tests are conducted to ensure quality, consistency in drug content between tablets, and that the drug will release and dissolve properly. Similar tests are described for other dosage forms like capsules, granules, powders, ointments, creams, suppositories, syrups, suspensions, emulsions, and novel delivery systems. The goal of evaluation is to quantify properties and assess interactions that impact drug stability and bioavailability.General considerations and method development in ce,



General considerations and method development in ce,ChowdaryPavani
Ěý
This document provides an overview of capillary electrophoresis (CE). It defines CE, describes its principle and instrumentation. CE involves separating components of a sample based on their differential rate of migration in an applied electric field. Key points covered include electrophoretic mobility, electroosmotic flow, sample introduction techniques, and common applications such as protein, DNA and pharmaceutical analysis. CE provides high resolution separations due to its small capillary diameter and long separation length.Residual solvent 



Residual solvent Saptarshi Das
Ěý
This document summarizes the determination of residual solvents in pharmaceuticals by gas chromatography according to ICH guidelines. Residual solvents are organic chemicals used in manufacturing that are not fully removed and may pose health risks. They are classified into Class 1, 2, or 3 based on toxicity, with Class 1 solvents prohibited. Gas chromatography with headspace injection and flame ionization detection is the primary analytical method. It involves using capillary columns, temperature programs, and identifies solvents by comparing retention times to standards. The document outlines accepted chromatographic conditions and procedures A, B, and C described in pharmacopeias.IMPURITIES AND STABILITY STUDIES



IMPURITIES AND STABILITY STUDIESprakash64742
Ěý
This document summarizes a seminar presentation on impurities and stability studies. It defines impurities as any component of a drug substance that is not the defined chemical entity. Impurities are classified as organic, inorganic, or residual solvents. Organic impurities can arise from manufacturing processes or storage and include starting materials, byproducts, and degradation products. Inorganic impurities result from manufacturing and include reagents, metals, and salts. Residual solvents are volatile organic chemicals used in drug substance synthesis. The document also discusses ICH guidelines for qualifying impurities based on safety testing and provides a decision tree for conducting safety studies of drug substances.IMPURITY PROFILING (SOURCES OF IMPURITIES)



IMPURITY PROFILING (SOURCES OF IMPURITIES)N Anusha
Ěý
The description, characterization and quantitation of identified and unidentified impurities present in the drug substances is known as impurity profile.
IMPURITIES in pharmaceuticals are unwanted chemicals, that even in small amounts may influence the efficacy and safety of the pharmaceutical products.
Degradation and Degradant Characterization



Degradation and Degradant CharacterizationGagan Deep
Ěý
This document discusses degradation, degradants, and degradant characterization. It defines degradation as changes in a substance's properties due to environmental factors or formulation interactions. Degradants are products formed from degradation that can reduce potency or induce toxicity. Degradant characterization involves profiling degradants using analytical techniques like LC-MS, GC-MS, and LC-NMR to separate and identify degradants. Factors like temperature, moisture, light exposure, pH changes, and microbes can induce degradation.Forced Degradation Study 



Forced Degradation Study Sumant Baukhandi
Ěý
This document outlines stability testing recommendations for different drug formulations, including whether acid-base, oxidative, photo-stability, thermal, or thermal-humidity testing is necessary or optional. It also describes how forced degradation studies are used to evaluate drug substance and product stability in various conditions, identify possible degradation pathways and products, and facilitate improvements to manufacturing and formulations.Study of Quality of Raw Materials and General methods of analysis of Raw mate...



Study of Quality of Raw Materials and General methods of analysis of Raw mate...Raghavendra institute of pharmaceutical education and research .
Ěý
Basics impurity profiling and degradent characterization[134]![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
![Basics impurity profiling and degradent characterization[134]](https://cdn.slidesharecdn.com/ss_thumbnails/basicsimpurityprofilinganddegradentcharacterization134-191014164210-thumbnail.jpg?width=560&fit=bounds)
Basics impurity profiling and degradent characterization[134]University Institute of Pharmaceutical Sciences
Ěý
Similar to MBTH FC-PDAB Reagents (20)
Unit 3 furan & thiophene



Unit 3 furan & thiopheneSowmiya Perinbaraj
Ěý
1) Furan and thiophene are 5-membered heterocyclic compounds containing oxygen and sulfur respectively. They are prepared via various synthetic routes and undergo electrophilic substitution and addition reactions.
2) Furan and thiophene have several medicinal uses. Furan derivatives like furosemide and ranitidine are used as diuretics and antiulcer drugs. Thiophene derivatives are used as antibiotics, antihistamines, anticonvulsants and anti-inflammatory drugs.
3) The document discusses the structures, properties, synthesis and reactions of furan and thiophene. It also outlines some of their common medicinal applications.Phenols and amines



Phenols and aminesOksana Kamenetska
Ěý
Lecture "Phenols and amines" on Pharmaceutical Chemistry is devoted by synthesis, physico-chemical properties and analysis of medicinal substances, which are derivatives of phenols and aromatic amines.Also is included examples of tests "KROK-2".APA seminar (1).pptx



APA seminar (1).pptxgurupavani
Ěý
This document discusses the use of MBTH and 2,4-DNP reagents for the estimation of drugs. MBTH is a chromogenic reagent that undergoes oxidative coupling reactions with phenols, amines, and aldehydes to form colored products. 2,4-DNP forms hydrazone precipitates with aldehydes and ketones. Factors like reagent concentration, pH, temperature, and oxidizing agent affect the reaction. Both reagents are useful for spectroscopic determination of various drugs in biological samples and industrial waste. However, 2,4-DNP is also toxic and hazardous if not handled carefully.Neutralization curves in acid base analytical titrations, indicators.



Neutralization curves in acid base analytical titrations, indicators.nehla313
Ěý
Neutralization curves in acid base analytical titrations, indicators,
strong acid strong base
weak acid strong bse
strong acid weak base
weak acid and weak basereagents



reagentsudaya rajitha
Ěý
Reagents and Reactions
This document discusses various reagents used for qualitative and quantitative analysis of pharmaceutical compounds. It describes common reagents like BM, MBTH, PDAB, and FC and their mechanisms of reaction. BM reacts via diazotization and coupling to form colored azo dyes. MBTH involves oxidation and coupling. PDAB acts through Schiff base formation. FC acts as an oxidizing/reducing agent. Example reactions and applications for analyzing drugs like paracetamol, nifidipine, and metronidazole are provided. In total, the document provides an overview of important reagents, reactions, mechanisms and uses for pharmaceutical analysis.Reagents & reactions in estimation of pharmaceuticals



Reagents & reactions in estimation of pharmaceuticalsudaya rajitha
Ěý
Reagents are used for the qualitative and quantitative determination of the pharmaceutical preparations, as they react with particular groups and increase their detectability by impart colour to the compounds.Displacement titration as analytical technique



Displacement titration as analytical techniqueP.K. Mani
Ěý
This document discusses displacement titration, specifically titrations of anions of weak acids (Bronsted bases) with strong acids. It provides examples of titrating salts of weak acids like sodium acetate, potassium cyanide, and borax with hydrochloric acid. The pH at the endpoint is calculated based on the concentration of the weak acid produced. Indicators that can be used for different titrations are also discussed based on the pH range at the endpoint.Analysis of diuretics



Analysis of diureticsbasha16111995
Ěý
based on these analysis of diuretics having an several methods to identifing thier analytical techniquesPA- I Acid-base titration (HRB) 



PA- I Acid-base titration (HRB) Harshadaa bafna
Ěý
This document discusses acid-base titration, including the theories and classification of acid-base indicators. It defines acids and bases, explaining that acids donate hydrogen ions and bases produce hydroxide ions. Acid-base titration is used to determine the concentration of acids or bases and involves neutralizing reactions between an acid and base. There are four main types of acid-base titrations classified by the strength of the acid and base involved. Indicator selection is also covered, with explanations of both the Ostwald and quinonoid theories of how indicators undergo color changes during titration.Option f4 f5



Option f4 f5Muhammad Yahaya
Ěý
Anthocyanins are naturally occurring pigments found in plants that give fruits and vegetables their red, blue, and purple colors. Their color stability is affected by pH levels - they are most stable and brightly colored at low pH. Higher temperatures cause the equilibrium of anthocyanins to shift, resulting in color loss. Carotenoids are pigments found in plants and algae that provide yellow to red colors. Their unsaturated bonds make them susceptible to oxidation from light, metals, and hydroperoxides, leading to color bleaching. Chlorophyll's color stability depends on pH levels - it is stable at basic pH but unstable in acidic conditions. Heating causes myoglobin in meat to auto-oxidize fromSynthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...![Synthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...](https://cdn.slidesharecdn.com/ss_thumbnails/d381519-170712065851-thumbnail.jpg?width=560&fit=bounds)
![Synthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...](https://cdn.slidesharecdn.com/ss_thumbnails/d381519-170712065851-thumbnail.jpg?width=560&fit=bounds)
![Synthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...](https://cdn.slidesharecdn.com/ss_thumbnails/d381519-170712065851-thumbnail.jpg?width=560&fit=bounds)
![Synthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...](https://cdn.slidesharecdn.com/ss_thumbnails/d381519-170712065851-thumbnail.jpg?width=560&fit=bounds)
Synthesis and antimicrobial activity of some methyl (4- (benzo[d]oxazol-2-yl)...QUESTJOURNAL
Ěý
1. A series of methyl (4-(benzo[d]oxazol-2-yl)phenyl) carbamodithioate amine derivatives were synthesized by reacting methyl (4-(benzo[d]oxazol-2-yl)phenyl) carbamodithioate with various amines.
2. The synthesized compounds were screened for their in vitro growth inhibiting activity against bacteria and fungi. Compounds exhibited moderate to high antibacterial and antifungal activity.
3. The structures of the compounds were characterized using spectral data such as IR, NMR, mass spectrometry, and elemental analysis. The compounds were then tested for their antimicrobial properties against various bacteria and fungi.Proteins,Fats determination



Proteins,Fats determinationRavi Vankudoth
Ěý
determination of proteins , fats and moisture in pharmaceutical preparation in pharmaceutical analysis department.Kto Lab Presentation



Kto Lab Presentationmuhamad norulakmal b ab malik
Ěý
laboratory presentation slide prepared by group 5 member. For inorganic chemistry laboratory course, SSC UTM skudai. we enjoy our presentation.Abts 2



Abts 2Yina Pajaro Gonzalez
Ěý
This document describes new methods for determining reactive bromine and chlorine species in water. It presents methods to analyze hypobromous acid (HOBr) and bromamines as a sum parameter, and to selectively determine hypochlorous acid (HOCl), monochloramine (NH2Cl), and chlorine dioxide (ClO2). The methods use 2,2-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) which reacts with the bromine and chlorine species to form a stable, green colored product that can be measured photometrically. The methods allow distinguishing between chlorine and chlorine dioxide without interference from chlorites, andPresentation FOR NAHEP CLASS1.pptx



Presentation FOR NAHEP CLASS1.pptxSayantikaBhattachary1
Ěý
This document provides information on various methods and reagents used for analyzing soil and water properties. It discusses methods for analyzing available nitrogen, phosphorus, potassium, calcium, magnesium and other nutrients in soil. It also outlines methods for analyzing phosphorus, sulfur, micronutrients and other elements. Further, it provides classifications for water quality based on electrical conductivity, sodium absorption ratio, residual sodium carbonate and chloride concentration.physicochemical and instrumental method analysis of pharmaceutical dosage forms,



physicochemical and instrumental method analysis of pharmaceutical dosage forms,Sharath Hns
Ěý
This document summarizes analytical methods for various drug classes including sulphonamides, barbiturates, adrenergic drugs, antitubercular drugs, and diuretics. It discusses physico-chemical properties and describes specific analytical techniques for several drugs including sulfadiazine, phenobarbital, xylometazoline hydrochloride, isoniazid, and furosemide. Methods include titrimetry, spectroscopy, chromatography, and colorimetry. The document serves as a reference for analytical techniques to identify and quantify pharmaceutical compounds.P h and buffer



P h and bufferPrincess Alen Aguilar-Cabunoc
Ěý
The document discusses pH, buffers, and buffer solutions. It begins by introducing pH and defining buffers. It then outlines the objectives, methodology, data and results of an experiment preparing phosphate and acetate buffer solutions. The results showed the buffers maintained pH when HCl was added. The discussion explains how buffers work to resist pH changes. It concludes that buffers are effective at maintaining pH within their given ranges, and understanding buffers and equations is crucial for preparation and analysis.Deepaknagargoje rc 2015



Deepaknagargoje rc 2015Mahatma Phule ASC College, Panvel
Ěý
This document summarizes a refresher course on chemistry that included a presentation on the ultrasound assisted one-pot synthesis of imidazole derivatives. Key points include:
- Imidazoles and benzimidazoles are important structures in drug chemistry, with examples like omeprazole cited.
- Literature methods for synthesis include using acids, metal chlorides, and other reagents.
- The presented method uses diethyl bromophosphate under ultrasonic irradiation to efficiently synthesize imidazole derivatives from benzil or benzoin, an aldehyde, and ammonium acetate in 30 minutes with yields of 90-98%.
- Optimization of reaction conditions like molar ratios,Spectrophotometric determination of some organophosphorus



Spectrophotometric determination of some organophosphorusRodica Dinica
Ěý
This document describes a spectrophotometric method for determining concentrations of three organophosphorus pesticides (diazinon, chlorpyrifos, and temefos) in pure form and in dosage forms. The method involves oxidizing the pesticides with cerium(IV) sulfate, then measuring the unreacted oxidizing agent by adding methyl orange and measuring absorbance. Variables such as reagent concentrations and order of addition were optimized. The method was validated and showed good linearity, precision, accuracy, and no interference from excipients. It provides a simple, sensitive, and low-cost way to determine concentrations of these pesticides.Phosphate Removal from Water by Iron-Mango Waste Powder.



Phosphate Removal from Water by Iron-Mango Waste Powder.implax
Ěý
Preparation of Fe(III) Loaded mango waste for the removal of phosphate from water. Its a dissertation.Recently uploaded (20)
BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAH



BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHcoacharyasetiyaki
Ěý
BISNIS BERKAH BERANGKAT KE MEKKAH ISTIKMAL SYARIAHEntity Framework Interview Questions PDF By ScholarHat



Entity Framework Interview Questions PDF By ScholarHatScholarhat
Ěý
Entity Framework Interview Questions PDF By ScholarHatHelping Autistic Girls Shine Webinar şÝşÝߣs



Helping Autistic Girls Shine Webinar şÝşÝߣsPooky Knightsmith
Ěý
For more information about my speaking and training work, visit: https://www.pookyknightsmith.com/speaking/How to create security group category in Odoo 17



How to create security group category in Odoo 17Celine George
Ěý
This slide will represent the creation of security group category in odoo 17. Security groups are essential for managing user access and permissions across different modules. Creating a security group category helps to organize related user groups and streamline permission settings within a specific module or functionality.Chapter 2. Strategic Management: Corporate Governance.pdf



Chapter 2. Strategic Management: Corporate Governance.pdfRommel Regala
Ěý
This course provides students with a comprehensive understanding of strategic management principles, frameworks, and applications in business. It explores strategic planning, environmental analysis, corporate governance, business ethics, and sustainability. The course integrates Sustainable Development Goals (SDGs) to enhance global and ethical perspectives in decision-making.RRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)



RRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)SONU HEETSON
Ěý
RRB ALP CBT 2 RAC Question Paper MCQ PDF Free Download. Railway Assistant Loco Pilot Mechanic Refrigeration and Air Conditioning Important Questions.How to Configure Proforma Invoice in Odoo 18 Sales



How to Configure Proforma Invoice in Odoo 18 SalesCeline George
Ěý
In this slide, we’ll discuss on how to configure proforma invoice in Odoo 18 Sales module. A proforma invoice is a preliminary invoice that serves as a commercial document issued by a seller to a buyer.Functional Muscle Testing of Facial Muscles.pdf



Functional Muscle Testing of Facial Muscles.pdfSamarHosni3
Ěý
Functional Muscle Testing of Facial Muscles.pdfComprehensive Guide to Antibiotics & Beta-Lactam Antibiotics.pptx



Comprehensive Guide to Antibiotics & Beta-Lactam Antibiotics.pptxSamruddhi Khonde
Ěý
📢 Comprehensive Guide to Antibiotics & Beta-Lactam Antibiotics
🔬 Antibiotics have revolutionized medicine, playing a crucial role in combating bacterial infections. Among them, Beta-Lactam antibiotics remain the most widely used class due to their effectiveness against Gram-positive and Gram-negative bacteria. This guide provides a detailed overview of their history, classification, chemical structures, mode of action, resistance mechanisms, SAR, and clinical applications.
📌 What You’ll Learn in This Presentation
âś… History & Evolution of Antibiotics
âś… Cell Wall Structure of Gram-Positive & Gram-Negative Bacteria
âś… Beta-Lactam Antibiotics: Classification & Subtypes
âś… Penicillins, Cephalosporins, Carbapenems & Monobactams
âś… Mode of Action (MOA) & Structure-Activity Relationship (SAR)
âś… Beta-Lactamase Inhibitors & Resistance Mechanisms
âś… Clinical Applications & Challenges.
🚀 Why You Should Check This Out?
Essential for pharmacy, medical & life sciences students.
Provides insights into antibiotic resistance & pharmaceutical trends.
Useful for healthcare professionals & researchers in drug discovery.
👉 Swipe through & explore the world of antibiotics today!
đź”” Like, Share & Follow for more in-depth pharma insights!Azure Administrator Interview Questions By ScholarHat



Azure Administrator Interview Questions By ScholarHatScholarhat
Ěý
Azure Administrator Interview Questions By ScholarHatHow to Configure Deliver Content by Email in Odoo 18 Sales



How to Configure Deliver Content by Email in Odoo 18 SalesCeline George
Ěý
In this slide, we’ll discuss on how to configure proforma invoice in Odoo 18 Sales module. A proforma invoice is a preliminary invoice that serves as a commercial document issued by a seller to a buyer.Effective Product Variant Management in Odoo 18



Effective Product Variant Management in Odoo 18Celine George
Ěý
In this slide we’ll discuss on the effective product variant management in Odoo 18. Odoo concentrates on managing product variations and offers a distinct area for doing so. Product variants provide unique characteristics like size and color to single products, which can be managed at the product template level for all attributes and variants or at the variant level for individual variants.Dr. Ansari Khurshid Ahmed- Factors affecting Validity of a Test.pptx



Dr. Ansari Khurshid Ahmed- Factors affecting Validity of a Test.pptxKhurshid Ahmed Ansari
Ěý
Validity is an important characteristic of a test. A test having low validity is of little use. Validity is the accuracy with which a test measures whatever it is supposed to measure. Validity can be low, moderate or high. There are many factors which affect the validity of a test. If these factors are controlled, then the validity of the test can be maintained to a high level. In the power point presentation, factors affecting validity are discussed with the help of concrete examples.AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...



AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...Prof. (Dr.) Vinod Kumar Kanvaria
Ěý
AI and Academic Writing, Short Term Course in Academic Writing and Publication, UGC-MMTTC, MANUU, 25/02/2025, Prof. (Dr.) Vinod Kumar Kanvaria, University of Delhi, vinodpr111@gmail.comAI and Academic Writing, Short Term Course in Academic Writing and Publicatio...



AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...Prof. (Dr.) Vinod Kumar Kanvaria
Ěý
MBTH FC-PDAB Reagents
- 2. MBTH ď‚— 3-Methyl-1,2-benzothiazoline hydrazone hydrochloride ď‚— Introduced in 1910 by BEST HORN ď‚— Used for estimation of no. of drugs having 1) phenols 2) aromatic amines 3) aldehydes 4) poly hydroxy compounds 5) indoles 6) carbazones 7) pheno thiazines
- 3. REACTION WITH PHENOLS ď‚— For the estimation of phenols the MBTH reagent is oxidised initially with an oxidising agent either FeCl3 or concentrated ammonium sulphate. ď‚— during oxidation MBTH looses 2 electrons and 1 proton. ď‚— The colours obtained by p- unsubstituted phenol is orange red where as p-substituted phenol gives violet green. REACTION WITH AMINES ď‚— MBTH reacts with amines in the similar way as it reacts with phenols and produces an intense blue colour.
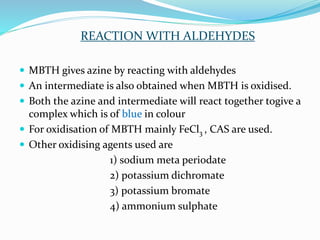
- 4. REACTION WITH ALDEHYDES ď‚— MBTH gives azine by reacting with aldehydes ď‚— An intermediate is also obtained when MBTH is oxidised. ď‚— Both the azine and intermediate will react together togive a complex which is of blue in colour ď‚— For oxidisation of MBTH mainly FeCl3 , CAS are used. ď‚— Other oxidising agents used are 1) sodium meta periodate 2) potassium dichromate 3) potassium bromate 4) ammonium sulphate
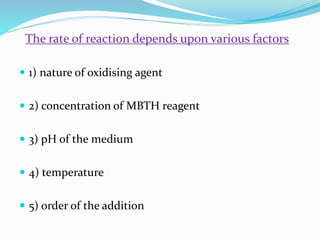
- 5. The rate of reaction depends upon various factors ď‚— 1) nature of oxidising agent ď‚— 2) concentration of MBTH reagent ď‚— 3) pH of the medium ď‚— 4) temperature ď‚— 5) order of the addition
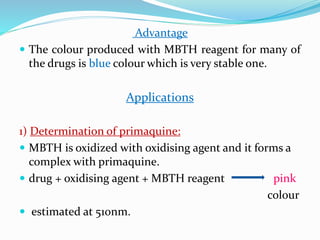
- 6. Advantage ď‚— The colour produced with MBTH reagent for many of the drugs is blue colour which is very stable one. Applications 1) Determination of primaquine: ď‚— MBTH is oxidized with oxidising agent and it forms a complex with primaquine. ď‚— drug + oxidising agent + MBTH reagent pink colour ď‚— estimated at 510nm.
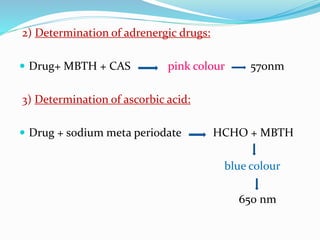
- 7. 2) Determination of adrenergic drugs: ď‚— Drug+ MBTH + CAS pink colour 570nm 3) Determination of ascorbic acid: ď‚— Drug + sodium meta periodate HCHO + MBTH blue colour 650 nm
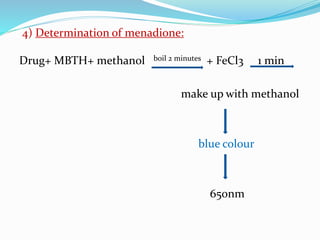
- 8. 4) Determination of menadione: Drug+ MBTH+ methanol boil 2 minutes + FeCl3 1 min make up with methanol blue colour 650nm
- 9. FC( Folin- Ciocalteau) REAGENT About the reagent: ď‚— Ortho phosphoric acid in combination with other acids like periodic, molybdic, vanidic, tungstic, molybdo tungstic acids will give a complex mixture known as hetero poly acid mixtures. ď‚— Treatment of such complexes with a reducing agent results corresponding product which is blue incolour. ď‚— E.g: phospho tungstate reducing agent tungstic blue
- 10. ď‚— The hetero poly acid mixture will give blue colour with a number of reducing agents. 1) stannous chloride 2) 1,2,4-amino napthol 3) sulphuric acid 4) ascorbic acid 5) hydrazine 6) thio sulphate 7) thio urea
- 11. Method of preparation: ď‚— Dissolve 10g of sodium tungstate and 2.5 g of sodium molybdate in 70ml of water. ď‚— Add 5ml of 85% phosphoric acid and 10ml conc. HCl ď‚— Reflux for 10min. ď‚— Add 16 g of lethium sulphate, 5 ml of water, add few drops of bromine. ď‚— The mixture is boiled for 15 minutes to remove excess bromine. ď‚— The solution is cooled, and diluted to 100ml and filtered.
- 12. Applications 1)Determination tyrosine: ď‚— Phenolic OH group is responsible for production of colour. 2) Determination of tryptophan: ď‚— indole group is responsible for production of colour 3) Determination of roxithromycin: ď‚— it reduces FC reagent in the presence of sodium carbonate to form blue coloured chromogen which can be measured at 760nm. 4) Determination of lansaprazole: ď‚— Drug + FC reagent blue colour 654nm
- 13. PDAB ď‚— Para di methyl amino benzaldehyde(ehrlich reagent) ď‚— This is useful for the determination of amines. ď‚— When the reagent reacts with amines azomethane is formed. ď‚— The azomethane is also known as schiffs base. ď‚— The reaction takes place in the presence of acids. ď‚— The acids used for the maintenance of acidic conditions are 1) para toluene sulphonic acid 2) tri chloro acetic acid 3) zinc chloride 4) phosphorous oxy chloride
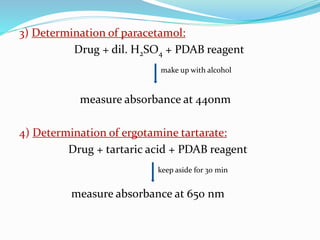
- 14. Applications 1)Determination of chlordiazepoxide: Drug + dil. acid + PDAB reagent + dilute with water measure absorbance at 450 nm 2) Determinaion of ethyl morphine HCl: Drug + dil. H2SO4 + PDAB reagent keep 1 hr at room temp Measure absorbance at 450 nm.
- 15. 3) Determination of paracetamol: Drug + dil. H2SO4 + PDAB reagent make up with alcohol measure absorbance at 440nm 4) Determination of ergotamine tartarate: Drug + tartaric acid + PDAB reagent keep aside for 30 min measure absorbance at 650 nm
- 16. 2,6- DICHLORO QUINONE CHLORIMIDE  Gibb’s reagent  Principle:  This reagent is capable of reacting with phenols which are unsubstituted in the para position to the phenolic hydroxil group to form 2,6-di chloro indo phenol which s blue in colour.





