Structured labeling for home use devices – assessment of the SPL format
- 1. 1 Structured labeling for home use devices – assessment of the SPL format Myron Finseth Principal Technical Writer Medtronic Cardiac Rhythm and Disease Management (CRDM) Technical Communications FDA Workshop, April 30, 2013 Accessible Medical Device Labeling in a Standard Content and Format
- 2. 2 Structured labeling for home use devices • Overview: – About Medtronic CRDM – HL7 Structured Product Labeling (SPL) format – SPL for home use devices – analysis – Considerations
- 3. 3 About Medtronic CRDM Cardiac Rhythm and Disease Management (CRDM) • Cardiac resynchronization therapy • Implantable cardiac defibrillators • Pacemakers • Heart monitors
- 4. 4 CRDM home use device labeling • Document types: – Clinician manual – Patient manual • Audiences: – Physicians, clinicians – Home healthcare nurses and aides – Patients – Regulatory authorities (US and International)
- 5. 5 HL7 Structured Product Labeling (SPL) format Data elements Content of labeling (text from manuals) HL7 SPL R5 data model Instructions for use Troubleshooting Warnings Precautions Contraindications Adverse events Manufacturer’s contact information Lot number /and serial number Special storage conditions Sterilization Contains latex Contain human tissue Controlled by lot / serial # Kit Combo product Controlled by Product exempt from DPM Package sterile? UDI Company name /address Contact information Brand name Model / version # GMDN Unit of use Device count Labeler company Storage and handling HL7 Health Informatics include data standards for Electronic Health Records, Patient Health Records, and mobile health information.
- 6. 6 • Reveal XT Patient Assistant – Patients use the device to mark points of time when they experience fainting spells. – Clinicians or home healthcare professionals train patients on how to use the device. – Medtronic REVEAL Patient Assistant 9538/9539 Clinician Manual used for training. Reveal XT Patient Assistant Reveal XT implantable heart monitor SPL for home use devices - analysis
- 7. 7 SPL for home use devices - analysis • Objectives: – Assess how the RTI study(1) recommendations align with Reveal Clinician manual. – Assess how the proposed UDI data elements support the text from the Reveal Clinician manual. – Better understand how a standardized labeling submission could impact device labeling. 1 Medical Device Labeling for Health Care Practitioners Focus Group Study, Final Report. Presented to Food and Drug Administration. Research Triangle Institute (RTI) International. 2011.
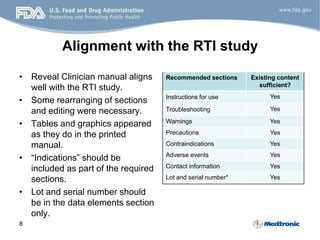
- 8. 8 Alignment with the RTI study • Reveal Clinician manual aligns well with the RTI study. • Some rearranging of sections and editing were necessary. • Tables and graphics appeared as they do in the printed manual. • “Indications” should be included as part of the required sections. • Lot and serial number should be in the data elements section only. Recommended sections Existing content sufficient? Instructions for use Yes Troubleshooting Yes Warnings Yes Precautions Yes Contraindications Yes Adverse events Yes Contact information Yes Lot and serial number* Yes
- 9. 9 Alignment with UDI data elements • The UDI data element connects the specific device to the correct labeling. • Including the data elements along with the content of labeling better represents content from existing manuals. • Makes sense to use device data elements in a similar way as drug and biologic products. – Support combination products – Maintain continuity in labeling for all regulated products. Highlighted data elements represent content from the existing Reveal manual.
- 10. 10 Implantable CRT device - patient manual • Patient manual is largely informational. Rather than “how to operate” a device, this manual informs “how to live” with their device. – Implant procedure and recovery – Registration, follow-up care, contact information – Precautions, medical procedures, EMI • Can be aligned closely to RTI sections and UDI data elements. • Possible use for distribution to electronic patient health record, including mobile device delivery. Active implantable devices represent the majority of CRDM products.
- 11. 11 Considerations • The RTI recommended sections do not add new content. The effort to implement would involve restructuring and editing existing content. • The HL7 SPL data model allows new opportunities for labeling distribution (dedicated website for devices, Health Information Systems, Patient Health Records, and mobile devices). • The required format needs to be a global solution. We recommend adoption through IMDRF (International Medical Device Regulators Forum) UDI Guidance.
- 12. 12 Considerations • Make the Labeling submission separate from the UDI submission and Device Listing submission. Perhaps, include SPL as part of the final labeling submission for PMA products and something similar for 510(k) submissions. • Industry needs the ability to choose or develop their own software to implement the requirement and sufficient time for deployment. • Some data elements lack clear definition (e.g., model / brand). Implementation of any solution requires that better definitions be developed and harmonized across all agency uses of those terms.