Metals ferrous and nonferrous
- 1. NON FERROUS METALS SUBMITTED BY: ASHISH KUMAR AVIRAL JAISWAL ROLI AGRAWAL SALIM IQBAL SANOVER GUPTA SHASHANK SINGH
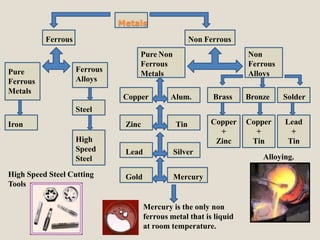
- 3. Metals Ferrous Non Ferrous Pure Ferrous Metals Ferrous Alloys Pure Non Ferrous Metals Non Ferrous Alloys Copper Alum. Zinc Tin Brass Bronze Solder Lead Silver Gold Mercury Mercury is the only non ferrous metal that is liquid at room temperature. Copper + Zinc Copper + Tin Lead + Tin Alloying. Iron Steel High Speed Steel High Speed Steel Cutting Tools
- 4. Ferrous Metals. Ferrous metals: Ferrous metals are metals that consist mostly of iron and small amounts of other elements. Ferrous metals are prone to rusting if exposed to moisture. Ferrous metals can also be picked up by a magnet. The rusting and magnetic properties in ferrous metals are both down due to the iron. Typical ferrous metals include mild steel, cast iron and steel. Examples: 1.Mild Steel. 2.Cast Iron. 3.High Carbon Steel. 4.High Speed Steel. 5.Stainless Steel. Rusting. Magnetism.
- 5. Non ŌĆō Ferrous Metal. Non-Ferrous Metals: Non-ferrous metals are metals that do not have any iron in them at all. This means that Non-ferrous metals are not attracted to a magnet and they also do not rust in the same way when exposed to moisture. Typical Non-ferrous metals include copper, aluminium (coke cans), tin and zinc. Examples: 1.Aluminium. 2.Copper. 3.Zinc. 4.Tin. 5.Lead. 6.Silver. 7.Gold. 8.Magnesium. Lead Tin Zinc
- 6. Non ŌĆō Ferrous Metal. Metal Type. Aluminium. It tends to be light in colour although it can be polished to a mirror like appearance. It is very light in weight. Metal Uses. Used for saucepans, cooking foil, window frames, ladders, expensive bicycles. Melting Point. 660┬░C
- 7. Non ŌĆō Ferrous Metal. Metal Type. Copper. It is a ductile and malleable metal. It is often red / brown in colour. It is a very good conductor of heat and electricity. Metal Uses. Used for plumbing, electric components, cookware and roof coverings. Melting Point. 1084┬░C
- 8. Non ŌĆō Ferrous Metal. Metal Type. Zinc. It is very resistant to corrosion from moisture. However zinc is a very weak metal and is used mainly for coating steel. Metal Uses. Used as a coating on screws, steel buckets etc It is also used to galvanize steel. Melting Point. 419┬░C
- 9. Non ŌĆō Ferrous Metal. Metal Type. Tin. It is a very ductile and very malleable metal. It is resistant to corrosion from moisture. It is bright silver in appearance. Tinplate is steel with a tin coating. Metal Uses. Used as a coating on food cans, beer cans. Used as whistles, tin foil and soldering. Melting Point. 231┬░C
- 10. Non ŌĆō Ferrous Metal. Metal Type. Lead. It is a soft, malleable metal. It is also counted as one of the heavy metals. Lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air. Metal Uses. Used for roof flashing. Also used for batteries and for X-ray protection. Lead is used for its weight in many ways. Melting Point. 327┬░C
- 11. Metal Type. Silver. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal. The metal occurs naturally in its pure, free form. Metal Uses. Used for jewelry and high quality cutlery. Also used for currency coins and sports trophies. Used in mirrors as a reflective metal. Melting Point. 961┬░C
- 12. Metal Type. Gold. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Gold resists attacks by individual acids It won't tarnish, discolor, crumble, or be affected by most solvents. Metal Uses. Used mainly for jewelry. Also used in computers as a conductor. Used for its reflective powers to protect satellites. Melting Point. 1337┬░C
- 13. Metal Type. Magnesium. Magnesium is a fairly strong, silvery- white, light-weight metal (one third lighter than aluminum) that slightly tarnishes when exposed to air. In a powder, this metal heats and ignites when exposed to moisture and burns with a white flame. Metal Uses. Magnesium is used in pyrotechnic (i.e. fireworks). It is alloyed with other metals to make them lighter and more easily welded. Melting Point. 648┬░C
- 14. Non ŌĆō Ferrous Metal Alloys. Non-Ferrous Metal Alloys: Non-ferrous metal alloys are metals that are a mixture of two or more metals. The main ones in everyday use are, Brass. Bronze. Solder. Heating metals in a furnace to form an alloy.
- 15. Non ŌĆō Ferrous Metal Alloys. Metal Type. Brass. Brass is a mixture of copper and zinc. Copper is the main component, and brass is usually classified as a copper alloy. The color of brass varies from a dark reddish brown to a light silvery yellow. Brass is stronger and harder than copper, but not as strong or hard as steel. It is easy to form into various shapes, a good conductor of heat, and generally resistant to corrosion from salt water. Metal Uses. Brass is used to make water fittings, screws, radiators, musical instruments, and cartridge casings for firearms. Melting Point. 940┬░C
- 16. Non ŌĆō Ferrous Metal Alloys. Metal Type. Bronze. Bronze is a metal alloy consisting primarily of copper, usually with tin as the main additive. It is a hard and brittle metal. It has a very high resistance to corrosion. Metal Uses. Used for ship propellers and underwater fittings. Also used for statues and medals. Melting Point. 950┬░C
- 17. Non ŌĆō Ferrous Metal Alloys. Metal Type. Solder. Solder is a fusible metal alloy used to join together metal work pieces and having a melting point below that of the work pieces. It is an alloy of Lead and Tin. Metal Uses. Solder is used for electronics, plumbing, jewelry making and repair processes where metal parts cannot be effectively or safely welded. Melting Point. 200┬░C
- 18. Metal pieces after mining and separation from their ores. (Note: Carbon and Phosphorous are non metals, while Silicon is a semi- metal) ChromiumCopper ManganeseNickel Phosphorous Molybdenum Silicon Carbon METAL PIECE
- 19. Metal Shapes. Metal can be provided in various shapes and sizes. Some examples of these are shown below. Round Solid. Round Hollow. (Tube) Square Solid. Square Hollow. (Box Iron) Hexagonal Solid. Hexagonal Hollow. Angle Iron Solid. Angle Iron Hollow.
- 20. Metals in Everyday Use. Below is a list of metals that would be used in the manufacturing of a bicycle.
- 21. ADVANTAGES ’éŚ Aluminum is one of the most frequently recycled non ferrous metals today and is also found in abundance in the earthŌĆÖs crust. It is the only material that covers the amount it costs to collect and process at a recycling centre. Recycling aluminum is economically viable, energy efficient and environmentally sound. ’éŚ All other metals are called ŌĆśnon-ferrousŌĆÖ and include aluminum, copper, zinc, lead, tin, nickel, magnesium, cobalt, silver and gold just to mention some of the more common ones. One of the reasons why there is a distinction between ferrous and non-ferrous metals is that ferrous materials are magnetic (with the important exception of stainless steel) and so can be separated off when scrap metal is collected and sorted in a scrap metal recycling yard.
- 22. DISADVANTAGES ’éŚ Nonferrous Metals ’éŚ Nonferrous metals are all alloys or metals that do not contain any iron. These metals are the opposite of ferrous metals, which are all metals that contain a percentage of iron. Unlike ferrous metals, nonferrous metals do not rust or oxidize. The only metal that is not considered nonferrous in the periodic table of elements is iron. A few examples of nonferrous metals are copper, tungsten steel, brass, chromium, titanium, nickel and aluminum. ’éŚ No Magnetic Attraction ’éŚ Unlike ferrous metals, nonferrous metals are not magnetically attractive. This can be a disadvantage since it excludes this metal from any application where magnetism is necessary or is an advantage. A few examples where the magnetic attraction of metals is used are in computer disc drives, automotive starters, audio speakers, microphone assemblies, some computer printers, and some vehicle motors. Nonferrous metals are useless in any of these applications because of the lack of magnetic attraction. ’éŚ Light-weight ’éŚ Nonferrous metals typically are light-weight and have limited strength capabilities. This prevents these metals from being used in any application where strength or heft is necessary. Because of this property, nonferrous metals are generally not used in industrial settings or industrial equipment. Nonferrous metals are also not typically used in decorative hardware or any types of tools or equipment. Because ferrous materials are stronger, they are typically used in industrial settings and areas where strength is important, such as in cast-iron fences and manhole covers. ’éŚ Cost ’éŚ On average, nonferrous metals cost more than ferrous metals, although the price can vary according to the metal. Industries or companies needing nonferrous metals for applications face a disadvantage compared to those companies using ferrous metals, because the cost is higher. The higher cost of metal can raise production costs for companies. For example, according to Earthworks Recycling, as of the time of publication, yellow brass, which is a nonferrous metal, costs $1.65 per pound. Iron, which is a ferrous metal, costs 35 cents per pound.
- 23. ELECTROPLATING ’éŚ Electroplating is a process that uses electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. The term is also used for electrical oxidation of anions on to a solid substrate, as in the formation of silver chloride on silver wire to make silver/silver-chloride electrodes. Electroplating is primarily used to change the surface properties of an object (such as abrasion and wear resistance, corrosion protection, lubricity, aesthetic qualities), but may also be used to build up thickness on undersized parts or to form objects by electroforming.
- 24. ANODISING ’éŚ Anodizing is an electrochemical process that converts the metal surface into a decorative, durable, corrosion-resistant, anodic oxide finish. Aluminum is ideally suited to anodizing, although other nonferrous metals, such as magnesium and titanium, also can be anodized.
- 25. THANK YOU