Microcytotoxicity
- 1. MICROCYTOTOXICITY ( microlymphocytotoxicity) Complement Dependent Cytotoxicity (CDC) By Dr Rania Abo-Shady Ass. Professor of Clinical Pathology Ain Shams University Fac. of Medicine
- 2. HLA - Typing SEROLOGY used to be the âgoldâ standard. Now being superseded by molecular techniques as they become more robust and time efficient. CELLULAR rarely used now. Originally used for Class II typing. MOLECULAR fast becoming the method of choice. Many laboratories test of choice.
- 3. SEROLOGY HLA Typing is done serologically by MICROCYTOTOXICITY which tests for complement mediated lysis of peripheral blood lymphocytes with a standard set of typing sera. Micro-cytotoxicity assay, utilizes serum with known anti-HLA antibodies that recognize particular HLA loci (HLA-A, HLA-B, HLA-C, HLA-DQ, HLA-DR /not DP) in order to match genetically similar individuals in hopes of performing a tissue transplantation. Viable peripheral blood lymphocytes are obtained by density gradient centrifugation using Ficoll at 19š- 22šC.
- 4. Cell isolation: -For HLA Class I typing: A mixture of T and B lymphocytes can be used by lymphocyte separation . -For HLA Class II typing: B lymphocytes are required. (Normal population 85- 90% T and 10-15% B cells) This can be achieved using a number of methods: *Magnetic beads Immunomagnetic bead separation is the current method of choice.It utilises polystyrene microspheres with a magnetisable core coated in monoclonal antibody for a HLA Class II b chain monomorphic epitope. Positive selection. *Nylon wool In the past neuraminidase treated sheep red blood cell rosetting and nylon wool have been used.
- 5. Coated with anti CD19 for B cells
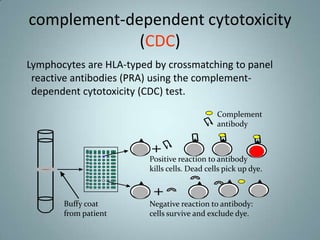
- 6. complement-dependent cytotoxicity (CDC) Lymphocytes are HLA-typed by crossmatching to panel reactive antibodies (PRA) using the complement- dependent cytotoxicity (CDC) test. Complement antibody Positive reaction to antibody kills cells. Dead cells pick up dye. Buffy coat Negative reaction to antibody: from patient cells survive and exclude dye.
- 7. Principle of Microlymphocytotoxic test Microlymphocytotoxic test: 3 stages âĒ 1.Viable lymphocytes are incubated with HLA specific antibodies. If the specific antigen is present on the cell the antibody is bound. âĒ 2.Rabbit serum as a source of complement is added, incubate. If antibody is bound to the HLA antigen on the cell surface it activates the complement which damages the cell membrane making it permeable to vital stains. âĒ 3.Results are visualised by adding dye usually a fluorochrome eg Ethidium Bromide although Eosin Y have been used in the past.
- 8. Cells are prelabelled with Fluorochrome before plating : when cells are killed +ve test ,fluorescine leaks out and a second fluorochrome a ethidium bromide is added to visualize dead cells.( double staining can be used by using a cocktail of Ethidium Bromide and Acridine Orange) If the reaction has taken place the EB or Eosin enters the cell and binds to the DNA. Test is left for 10 minutes and then read using an inverted fluorescent microscope.
- 9. Terazaki plate and inverted microscopy
- 10. Negative (living cells) Positive (dead cells)
- 11. The percentage of stained cells in each well is used to determine whether the cells are positive or negative for the Positive Negative HLA antigen.
- 12. The Score Values in Lymphocytotoxicity Test (Terasaki and McClelland, 1964) The percentage of Score Evaluation dead cells 0-10 1 Negative 11-20 2 doubtful positive/Negative 21-50 4 Weak Positive(++) 51-80 6 Positive(+++) 81-100 8 Strong positive(++++) The number of dead (lysed) lymphocytes was compared with the total number of cells quoted as score values
- 13. Advantages: âĒ Easily performed does not require expensive equipment. âĒ Takes around three hours to perform âĒ Low level resolution, with good antisera gives reliable results Disadvantages: âĒ Requires large volumes of blood âĒ Requires viable lymphocytes âĒ Difficult to find good antisera for rarer antigens in different populations