MixturesandPureSubstances.ppt
Download as ppt, pdf0 likes41 views
An atom is to an element as a single unit is to a pure substance. An atom is to a molecule as a single unit is to multiple units bound together. An atom is to a compound molecule as a single unit is to multiple different units bound together. Elements are pure substances made of single types of atoms. Molecules are multiple atoms of one or more elements bound together, such as in compounds. Compounds are made of two or more different elements chemically bonded together at the molecular level. Atoms are the fundamental unit that make up elements, and atoms can bond together to form molecules, such as in compounds.
1 of 21
Download to read offline














Ad
Recommended
MixturesandPureSubstances.ppt
MixturesandPureSubstances.pptJennifer873722
╠²
An atom is the basic unit of an element. Elements are pure substances made of only one type of atom.
A molecule is formed when two or more atoms of one or more elements are chemically bonded together. Compounds are pure substances made of molecules containing two or more elements chemically bonded together in a fixed ratio.
So in summary:
- An atom is to an element as a single unit is to the whole substance
- An atom is to a molecule as a single unit is to a combined group of atoms
- An atom is to a compound molecule as a single unit is to a combined group of different atoms bonded together.
Elements are made of single atoms while compounds and molecules involve multiple atoms ofMixtures and Pure Substances.ppt
Mixtures and Pure Substances.pptArmindaSagampod2
╠²
An atom is the basic unit of an element. Elements are pure substances made of only one type of atom.
A molecule is formed when two or more atoms of one or more elements are chemically bonded together. Compounds are pure substances made of molecules containing two or more elements chemically bonded together in a fixed ratio.
So in summary:
- An atom is to an element as the basic unit is to the pure substance made of that unit.
- An atom is to a molecule as the individual unit is to the combined structure formed from two or more bonded units.
- An atom is to a compound molecule as the individual unit is to the combined structure formed from two or more different bonded units that makeMixtures and Pure Substances.ppt0987788768768
Mixtures and Pure Substances.ppt0987788768768ElliePamaPastrana
╠²
The document explains the differences between pure substances and mixtures, detailing how elements and compounds qualify as pure substances and the characteristics of heterogeneous and homogeneous mixtures. It discusses solubility, methods for separating mixtures such as sedimentation and distillation, and provides various examples to illustrate these concepts. Additionally, it emphasizes the relationships between atoms, elements, molecules, and compounds.MixturesandPureSubstances.ppt
MixturesandPureSubstances.pptMaryGraceVilbarSanti
╠²
Here are the key differences between pure substances and mixtures:
- Pure substances are either elements or compounds, which have a definite composition and properties. Mixtures can be composed of any substances.
- Elements are made of only one type of atom. Compounds are made of two or more elements chemically combined in a fixed ratio. Mixtures may contain multiple elements without fixed ratios.
- Particles in pure substances are molecules or atoms of a single type. Particles in mixtures can be molecules, atoms, or even larger phases that have not undergone a chemical reaction with each other.
- Pure substances have a consistent melting and boiling point as well as other properties. Mixtures can have varying properties depending on the relative amountsMixturesandPureSubstances (1).ppt
MixturesandPureSubstances (1).pptMarkAnthonyCabulay1
╠²
An atom is the basic unit of an element, just as an element is the basic unit of a compound.
An atom is to an element as a single unit is to the simplest substance. An element consists of multiple identical atoms bonded together.
An atom is to a molecule as a single unit is to multiple units. A molecule is formed when two or more different atoms bond together chemically, whereas an atom is a single unit of an element.
An atom is to a compound molecule as a single unit is to multiple different units. A compound is made of two or more different elements chemically bonded together to form a new substance, with a compound molecule consisting of multiple different bonded atoms.
In summary, atoms areElements, Compounds & Mixtures Spring 2014
Elements, Compounds & Mixtures Spring 2014jmori
╠²
This document provides instruction for classroom activities and assignments. It includes:
1. A list of materials needed for an assignment on Bohr models, Lewis dot diagrams, and mixtures due today.
2. Notification that any missing work is due today and will be graded over the weekend.
3. Details on an upcoming quick write assignment where students can use references and will self-grade with colored pencils.
4. Information for students assigned to lead an upcoming discussion on elements, compounds, and mixtures using a slideshow.
So in summary, the document outlines materials and tasks due, guidelines for an assignment, and instructions for a student-led classroom discussion.MixturesandPureSubstances.ppt
MixturesandPureSubstances.pptMAHAZELTEOLOGO3
╠²
Elements are pure substances that cannot be broken down further, consisting of atoms of a single type. Compounds are pure substances made of two or more elements chemically bonded together as molecules. Mixtures contain elements or compounds mixed together but not chemically bonded. Heterogeneous mixtures are not uniform throughout, while homogeneous mixtures and solutions appear uniform with particles distributed evenly throughout.Physical Properties of Pure Substances -mixtures and_solutions - L4.ppt
Physical Properties of Pure Substances -mixtures and_solutions - L4.pptKhomotsoVincentMokob
╠²
Physical properties of Pure SubstancesCBSE Class IX SCIENCE CHEMISTRY Is matter around us pure
CBSE Class IX SCIENCE CHEMISTRY Is matter around us purePranav Ghildiyal
╠²
The document provides an overview of matter, defining it as any substance with mass and volume, existing in various states including solid, liquid, gas, and plasma. It discusses mixtures and their types, such as homogeneous and heterogeneous mixtures, along with solutions, suspensions, and colloids, highlighting their properties and differences. Additionally, it explains pure substances, elements, and compounds, emphasizing the unique characteristics and behavior of these forms of matter.Classification of Matter (General Chemistry)
Classification of Matter (General Chemistry)merielleshaned
╠²
The document discusses the classification of matter, focusing on the differences between homogeneous and heterogeneous mixtures, as well as between pure substances and mixtures, elements, and compounds. It explains various separation techniques, such as filtration, evaporation, chromatography, and distillation, and highlights that pure substances cannot be separated by physical means. The document also includes an assignment for examining household materials to identify their elements and compounds.Elements, Compounds & Mixtures spring 2014. Day 2
Elements, Compounds & Mixtures spring 2014. Day 2jmori
╠²
This document provides instructions and information about elements, compounds, and mixtures. It begins by listing materials needed and notes about assignments. It then defines elements as pure substances made of one type of atom. Compounds are made of two or more types of atoms chemically bonded in a set ratio. Mixtures contain two or more elements or compounds blended together physically with no set ratio. Solutions are homogeneous mixtures where a solute is dissolved evenly in a solvent. Saturation levels depend on how much solute can dissolve in a given amount of solvent at a certain temperature. In the end, it reviews what was learned about elements, compounds and mixtures, and notes assignments that were due.Is Matter Around us Pure
Is Matter Around us PureKrishnaDhaked
╠²
This chemistry document outlines learning objectives and content for a Grade 9 chemistry unit on matter and mixtures. The key learning objectives are for students to be able to classify matter, distinguish between pure substances and mixtures, understand different types of mixtures and separation techniques, and demonstrate physical and chemical changes. The document provides explanations of pure substances, elements, compounds, mixtures and different types of mixtures. It also describes various separation techniques including evaporation, filtration, sublimation, centrifugation and chromatography.Elements, Compounds & Mixtures spring 2014. Day 2
Elements, Compounds & Mixtures spring 2014. Day 2jmori
╠²
This document provides instructions for students regarding an assignment on elements, compounds, and mixtures. It includes a list of materials needed, project grades, discussion leading responsibilities, and content on the topics. Key points:
- Students are to log into an online learning platform, open a slide presentation on elements/compounds/mixtures, and complete pages 1-2 of assigned readings.
- The content discusses the defining characteristics of elements, compounds, and mixtures - namely that elements are pure with one type of atom, compounds are pure with two or more types of atoms bonded in a set ratio, and mixtures are not pure with no set ratio.
- Examples of each are given, along with explanations of solutions, saturation levelsPower Notes Elements, Compounds and Mixtures- Day 2
Power Notes Elements, Compounds and Mixtures- Day 2jmori
╠²
The document provides instructions for students on completing assignments related to elements, compounds, and mixtures, including taking a corrected test, finishing Power Notes on these topics, and turning in Cornell notes. Students are asked to identify and classify examples of elements, compounds, and mixtures using illustrations. The final pages define solutions and saturation levels, asking students to provide their own examples of each.Elements Compounds Mixtures
Elements Compounds MixturesChristine Onwenu
╠²
Matter can be classified into mixtures, elements, and compounds based on its composition. Mixtures consist of two or more substances that can be separated by physical means, whereas elements are pure substances that cannot be broken down further. Compounds are pure substances formed from two or more elements that can be separated by chemical means.Elements compounds mixtures
Elements compounds mixturesDee Bayn
╠²
Scientists classify matter based on its composition into mixtures, elements, and compounds. Mixtures are two or more substances that are not chemically combined and can be separated physically. Elements are the simplest substances that cannot be broken down further, while compounds are pure substances made of two or more elements chemically bonded together. Mixtures can be further classified as homogeneous if the substances are uniformly mixed throughout, or heterogeneous if they are not. Solutions are homogeneous mixtures where one substance dissolves in another.elements_compounds_mixtureschemistry.ppt
elements_compounds_mixtureschemistry.pptRilfiHelmanda1
╠²
Matter can be classified into mixtures, elements, and compounds based on its composition, where mixtures consist of two or more substances that are not chemically combined, while elements are pure substances that cannot be broken down, and compounds are formed from two or more elements. Homogeneous and heterogeneous mixtures differ in uniformity and the ability to be separated, with homogeneous mixtures appearing consistent throughout. Solutions are a type of homogeneous mixture where one substance dissolves in another, exemplifying the best-mixed variety of matter.elements_compounds_mixtures.ppt, science
elements_compounds_mixtures.ppt, scienceAizaRazonado
╠²
Matter can be classified into three categories: mixtures, elements, and compounds, based on composition and properties. Mixtures can be homogeneous or heterogeneous, while elements are the simplest form of pure substances and compounds are combinations of elements that can be broken down chemically. Understanding these classifications helps scientists analyze and separate different types of matter effectively.Elements, Compounds & Mixtures Day 2 fall 2012
Elements, Compounds & Mixtures Day 2 fall 2012jmori
╠²
This document provides instructions and questions for a lesson on elements, compounds, and mixtures. It includes a list of materials needed, such as a pencil and textbook. It also lists test retake opportunities and instructions for leading a discussion on the topic. The document contains questions to guide student understanding of key concepts like the difference between elements and compounds, properties of mixtures, and examples of solutes, solvents, and solutions.Pure_Substances_mixtures_and_solutions_2017.ppt
Pure_Substances_mixtures_and_solutions_2017.pptJessahMaeRPrincesa
╠²
Pure substances like elements and compounds have a definite chemical composition and cannot be separated into simpler substances using only physical processes. Mixtures, on the other hand, are combinations of substances that are not chemically bonded and can be separated physically. Mixtures can be either heterogeneous, with distinguishable parts, or homogeneous, appearing uniform throughout. Solutions are a type of homogeneous mixture consisting of a solute dissolved evenly in a solvent.Pure_Substances_mixtures_and_solutions_2017.ppt
Pure_Substances_mixtures_and_solutions_2017.pptJessahMaeRPrincesa
╠²
Pure substances like elements and compounds have a definite chemical composition and cannot be separated into simpler substances using only physical processes. Mixtures, on the other hand, are combinations of substances that are not chemically bonded and can be separated physically. Mixtures can be either heterogeneous, with distinguishable parts, or homogeneous, appearing uniform throughout. Solutions represent a special type of homogeneous mixture formed by dissolving one or more substances (solutes) into another (solvent).CSEC Chemistry Review - Mixtures and Compounds
CSEC Chemistry Review - Mixtures and CompoundsKevin Small
╠²
The document reviews the concepts of pure substances, mixtures, and compounds in chemistry, highlighting their definitions and differences. It details various separation techniques including decantation, filtration, evaporation, crystallization, separating funnel, chromatography, and distillation. Overall, the document serves as a study guide for identifying and understanding these fundamental chemical principles and methods.Elements,compounds and mixtures
Elements,compounds and mixturesDebjaniPurkayastha1
╠²
Elements are pure substances that cannot be broken down further by chemical reactions or processes. They consist of only one type of atom and exist as either individual atoms or molecules made of atoms of the same element. Compounds are pure substances made of two or more elements chemically bonded together in fixed ratios. Compounds have distinct properties and can be broken down into their constituent elements. Mixtures are physical combinations of elements or compounds not chemically bonded. They do not have a fixed composition and their properties depend on the substances that make them up. Mixtures can be separated into their components using physical processes like filtration or evaporation.Elements, Compounds & Mixtures Day 2
Elements, Compounds & Mixtures Day 2jmori
╠²
1. The document provides instructions for an assignment on elements, compounds, and mixtures. It includes tasks to complete notes on pages 1 and 2 of the assigned reading, create a cover page for Binder Check #2, and print the Table of Contents.
2. Key concepts are defined, including that elements are pure substances made of one atom, compounds contain two or more elements in a fixed ratio, and mixtures have no fixed ratio and can be separated physically.
3. Examples are provided of particles in elements, compounds, and mixtures. Solutions are also discussed, where the solute dissolves in the solvent, and saturation levels are defined.Elements, compounds & mixtures day 2
Elements, compounds & mixtures day 2jmori
╠²
1. The document provides instructions for an assignment on elements, compounds, and mixtures. It includes tasks to complete notes on pages 1 and 2 of the assigned reading, create a cover page for Binder Check #2, and print the Table of Contents.
2. Key concepts are defined, including that elements are pure substances made of one atom, compounds contain two or more elements in a fixed ratio, and mixtures have no fixed ratio and can be separated physically.
3. Examples are provided of particles in elements, compounds, and mixtures. Solutions are also discussed, where the solute dissolves in the solvent, and saturation levels are defined.INTODUCTION TO CHEMISTRY PHARMACY FIRST YEAR.pptx
INTODUCTION TO CHEMISTRY PHARMACY FIRST YEAR.pptxAndrewSilungwe2
╠²
Chemistry is the study of matter and its properties. The document outlines the core topics covered in a chemistry course including atomic structure, the periodic table, bonding, nomenclature, chemical reactions, stoichiometry, gas laws, solutions, acids and bases, and thermochemistry. It defines matter as anything that has mass and takes up space, and describes the two main types of matter as pure substances and mixtures. Pure substances are either elements, which consist of only one type of atom, or compounds, made of two or more bonded elements. Mixtures can be solutions, mechanical mixtures, suspensions, or colloids depending on whether the parts are evenly mixed or separated.Elements, Compounds & Mixtures Day 2 fall 2012
Elements, Compounds & Mixtures Day 2 fall 2012jmori
╠²
This document provides instructions and questions for a class discussion on elements, compounds, and mixtures. It includes a list of materials needed, test retake opportunities, and assignment updates. It also provides 21 multiple choice questions to be discussed covering topics like families of elements, the periodic table, properties of mixtures, solutions, and examples of homogeneous and heterogeneous mixtures. The document concludes by outlining the discussion steps and assigning reading from the textbook on elements, compounds and mixtures.CHM1 11_12 Q1 0103 PF FD.pptx
CHM1 11_12 Q1 0103 PF FD.pptxDGarcia20
╠²
This document provides an overview of classifying matter as either pure substances or mixtures. Pure substances are either elements or compounds, with elements made of a single type of atom and compounds made of multiple different atoms bonded chemically. Mixtures can be either homogeneous, with components evenly mixed on a microscopic level, or heterogeneous, with distinguishable components. The document defines key terms and provides examples to differentiate pure substances from mixtures and their various sub-classifications.ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptx
ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptxBelicia R.S
╠²
Role play : First Aid- CPR, Recovery position and Hand hygiene.
Scene 1: Three friends are shopping in a mall
Scene 2: One of the friend becomes victim to electric shock.
Scene 3: Arrival of a first aider
Steps:
Safety First
Evaluate the victimŌĆśs condition
Call for help
Perform CPR- Secure an open airway, Chest compression, Recuse breaths.
Put the victim in Recovery position if unconscious and breathing normally.
More Related Content
Similar to MixturesandPureSubstances.ppt (20)
CBSE Class IX SCIENCE CHEMISTRY Is matter around us pure
CBSE Class IX SCIENCE CHEMISTRY Is matter around us purePranav Ghildiyal
╠²
The document provides an overview of matter, defining it as any substance with mass and volume, existing in various states including solid, liquid, gas, and plasma. It discusses mixtures and their types, such as homogeneous and heterogeneous mixtures, along with solutions, suspensions, and colloids, highlighting their properties and differences. Additionally, it explains pure substances, elements, and compounds, emphasizing the unique characteristics and behavior of these forms of matter.Classification of Matter (General Chemistry)
Classification of Matter (General Chemistry)merielleshaned
╠²
The document discusses the classification of matter, focusing on the differences between homogeneous and heterogeneous mixtures, as well as between pure substances and mixtures, elements, and compounds. It explains various separation techniques, such as filtration, evaporation, chromatography, and distillation, and highlights that pure substances cannot be separated by physical means. The document also includes an assignment for examining household materials to identify their elements and compounds.Elements, Compounds & Mixtures spring 2014. Day 2
Elements, Compounds & Mixtures spring 2014. Day 2jmori
╠²
This document provides instructions and information about elements, compounds, and mixtures. It begins by listing materials needed and notes about assignments. It then defines elements as pure substances made of one type of atom. Compounds are made of two or more types of atoms chemically bonded in a set ratio. Mixtures contain two or more elements or compounds blended together physically with no set ratio. Solutions are homogeneous mixtures where a solute is dissolved evenly in a solvent. Saturation levels depend on how much solute can dissolve in a given amount of solvent at a certain temperature. In the end, it reviews what was learned about elements, compounds and mixtures, and notes assignments that were due.Is Matter Around us Pure
Is Matter Around us PureKrishnaDhaked
╠²
This chemistry document outlines learning objectives and content for a Grade 9 chemistry unit on matter and mixtures. The key learning objectives are for students to be able to classify matter, distinguish between pure substances and mixtures, understand different types of mixtures and separation techniques, and demonstrate physical and chemical changes. The document provides explanations of pure substances, elements, compounds, mixtures and different types of mixtures. It also describes various separation techniques including evaporation, filtration, sublimation, centrifugation and chromatography.Elements, Compounds & Mixtures spring 2014. Day 2
Elements, Compounds & Mixtures spring 2014. Day 2jmori
╠²
This document provides instructions for students regarding an assignment on elements, compounds, and mixtures. It includes a list of materials needed, project grades, discussion leading responsibilities, and content on the topics. Key points:
- Students are to log into an online learning platform, open a slide presentation on elements/compounds/mixtures, and complete pages 1-2 of assigned readings.
- The content discusses the defining characteristics of elements, compounds, and mixtures - namely that elements are pure with one type of atom, compounds are pure with two or more types of atoms bonded in a set ratio, and mixtures are not pure with no set ratio.
- Examples of each are given, along with explanations of solutions, saturation levelsPower Notes Elements, Compounds and Mixtures- Day 2
Power Notes Elements, Compounds and Mixtures- Day 2jmori
╠²
The document provides instructions for students on completing assignments related to elements, compounds, and mixtures, including taking a corrected test, finishing Power Notes on these topics, and turning in Cornell notes. Students are asked to identify and classify examples of elements, compounds, and mixtures using illustrations. The final pages define solutions and saturation levels, asking students to provide their own examples of each.Elements Compounds Mixtures
Elements Compounds MixturesChristine Onwenu
╠²
Matter can be classified into mixtures, elements, and compounds based on its composition. Mixtures consist of two or more substances that can be separated by physical means, whereas elements are pure substances that cannot be broken down further. Compounds are pure substances formed from two or more elements that can be separated by chemical means.Elements compounds mixtures
Elements compounds mixturesDee Bayn
╠²
Scientists classify matter based on its composition into mixtures, elements, and compounds. Mixtures are two or more substances that are not chemically combined and can be separated physically. Elements are the simplest substances that cannot be broken down further, while compounds are pure substances made of two or more elements chemically bonded together. Mixtures can be further classified as homogeneous if the substances are uniformly mixed throughout, or heterogeneous if they are not. Solutions are homogeneous mixtures where one substance dissolves in another.elements_compounds_mixtureschemistry.ppt
elements_compounds_mixtureschemistry.pptRilfiHelmanda1
╠²
Matter can be classified into mixtures, elements, and compounds based on its composition, where mixtures consist of two or more substances that are not chemically combined, while elements are pure substances that cannot be broken down, and compounds are formed from two or more elements. Homogeneous and heterogeneous mixtures differ in uniformity and the ability to be separated, with homogeneous mixtures appearing consistent throughout. Solutions are a type of homogeneous mixture where one substance dissolves in another, exemplifying the best-mixed variety of matter.elements_compounds_mixtures.ppt, science
elements_compounds_mixtures.ppt, scienceAizaRazonado
╠²
Matter can be classified into three categories: mixtures, elements, and compounds, based on composition and properties. Mixtures can be homogeneous or heterogeneous, while elements are the simplest form of pure substances and compounds are combinations of elements that can be broken down chemically. Understanding these classifications helps scientists analyze and separate different types of matter effectively.Elements, Compounds & Mixtures Day 2 fall 2012
Elements, Compounds & Mixtures Day 2 fall 2012jmori
╠²
This document provides instructions and questions for a lesson on elements, compounds, and mixtures. It includes a list of materials needed, such as a pencil and textbook. It also lists test retake opportunities and instructions for leading a discussion on the topic. The document contains questions to guide student understanding of key concepts like the difference between elements and compounds, properties of mixtures, and examples of solutes, solvents, and solutions.Pure_Substances_mixtures_and_solutions_2017.ppt
Pure_Substances_mixtures_and_solutions_2017.pptJessahMaeRPrincesa
╠²
Pure substances like elements and compounds have a definite chemical composition and cannot be separated into simpler substances using only physical processes. Mixtures, on the other hand, are combinations of substances that are not chemically bonded and can be separated physically. Mixtures can be either heterogeneous, with distinguishable parts, or homogeneous, appearing uniform throughout. Solutions are a type of homogeneous mixture consisting of a solute dissolved evenly in a solvent.Pure_Substances_mixtures_and_solutions_2017.ppt
Pure_Substances_mixtures_and_solutions_2017.pptJessahMaeRPrincesa
╠²
Pure substances like elements and compounds have a definite chemical composition and cannot be separated into simpler substances using only physical processes. Mixtures, on the other hand, are combinations of substances that are not chemically bonded and can be separated physically. Mixtures can be either heterogeneous, with distinguishable parts, or homogeneous, appearing uniform throughout. Solutions represent a special type of homogeneous mixture formed by dissolving one or more substances (solutes) into another (solvent).CSEC Chemistry Review - Mixtures and Compounds
CSEC Chemistry Review - Mixtures and CompoundsKevin Small
╠²
The document reviews the concepts of pure substances, mixtures, and compounds in chemistry, highlighting their definitions and differences. It details various separation techniques including decantation, filtration, evaporation, crystallization, separating funnel, chromatography, and distillation. Overall, the document serves as a study guide for identifying and understanding these fundamental chemical principles and methods.Elements,compounds and mixtures
Elements,compounds and mixturesDebjaniPurkayastha1
╠²
Elements are pure substances that cannot be broken down further by chemical reactions or processes. They consist of only one type of atom and exist as either individual atoms or molecules made of atoms of the same element. Compounds are pure substances made of two or more elements chemically bonded together in fixed ratios. Compounds have distinct properties and can be broken down into their constituent elements. Mixtures are physical combinations of elements or compounds not chemically bonded. They do not have a fixed composition and their properties depend on the substances that make them up. Mixtures can be separated into their components using physical processes like filtration or evaporation.Elements, Compounds & Mixtures Day 2
Elements, Compounds & Mixtures Day 2jmori
╠²
1. The document provides instructions for an assignment on elements, compounds, and mixtures. It includes tasks to complete notes on pages 1 and 2 of the assigned reading, create a cover page for Binder Check #2, and print the Table of Contents.
2. Key concepts are defined, including that elements are pure substances made of one atom, compounds contain two or more elements in a fixed ratio, and mixtures have no fixed ratio and can be separated physically.
3. Examples are provided of particles in elements, compounds, and mixtures. Solutions are also discussed, where the solute dissolves in the solvent, and saturation levels are defined.Elements, compounds & mixtures day 2
Elements, compounds & mixtures day 2jmori
╠²
1. The document provides instructions for an assignment on elements, compounds, and mixtures. It includes tasks to complete notes on pages 1 and 2 of the assigned reading, create a cover page for Binder Check #2, and print the Table of Contents.
2. Key concepts are defined, including that elements are pure substances made of one atom, compounds contain two or more elements in a fixed ratio, and mixtures have no fixed ratio and can be separated physically.
3. Examples are provided of particles in elements, compounds, and mixtures. Solutions are also discussed, where the solute dissolves in the solvent, and saturation levels are defined.INTODUCTION TO CHEMISTRY PHARMACY FIRST YEAR.pptx
INTODUCTION TO CHEMISTRY PHARMACY FIRST YEAR.pptxAndrewSilungwe2
╠²
Chemistry is the study of matter and its properties. The document outlines the core topics covered in a chemistry course including atomic structure, the periodic table, bonding, nomenclature, chemical reactions, stoichiometry, gas laws, solutions, acids and bases, and thermochemistry. It defines matter as anything that has mass and takes up space, and describes the two main types of matter as pure substances and mixtures. Pure substances are either elements, which consist of only one type of atom, or compounds, made of two or more bonded elements. Mixtures can be solutions, mechanical mixtures, suspensions, or colloids depending on whether the parts are evenly mixed or separated.Elements, Compounds & Mixtures Day 2 fall 2012
Elements, Compounds & Mixtures Day 2 fall 2012jmori
╠²
This document provides instructions and questions for a class discussion on elements, compounds, and mixtures. It includes a list of materials needed, test retake opportunities, and assignment updates. It also provides 21 multiple choice questions to be discussed covering topics like families of elements, the periodic table, properties of mixtures, solutions, and examples of homogeneous and heterogeneous mixtures. The document concludes by outlining the discussion steps and assigning reading from the textbook on elements, compounds and mixtures.CHM1 11_12 Q1 0103 PF FD.pptx
CHM1 11_12 Q1 0103 PF FD.pptxDGarcia20
╠²
This document provides an overview of classifying matter as either pure substances or mixtures. Pure substances are either elements or compounds, with elements made of a single type of atom and compounds made of multiple different atoms bonded chemically. Mixtures can be either homogeneous, with components evenly mixed on a microscopic level, or heterogeneous, with distinguishable components. The document defines key terms and provides examples to differentiate pure substances from mixtures and their various sub-classifications.Recently uploaded (20)
ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptx
ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptxBelicia R.S
╠²
Role play : First Aid- CPR, Recovery position and Hand hygiene.
Scene 1: Three friends are shopping in a mall
Scene 2: One of the friend becomes victim to electric shock.
Scene 3: Arrival of a first aider
Steps:
Safety First
Evaluate the victimŌĆśs condition
Call for help
Perform CPR- Secure an open airway, Chest compression, Recuse breaths.
Put the victim in Recovery position if unconscious and breathing normally.
Overview of Employee in Odoo 18 - Odoo ║▌║▌▀Żs
Overview of Employee in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
The employee module is a core component of the HR workspace that helps the business to get the employee activities and details. This would also allow you to get the employee details by acting as a centralized system and accessing, updating, and managing all the other employee data. THERAPEUTIC COMMUNICATION included definition, characteristics, nurse patient...
THERAPEUTIC COMMUNICATION included definition, characteristics, nurse patient...parmarjuli1412
╠²
The document provides an overview of therapeutic communication, emphasizing its importance in nursing to address patient needs and establish effective relationships. THERAPEUTIC COMMUNICATION included some topics like introduction of COMMUNICATION, definition, types, process of communication, definition therapeutic communication, goal, techniques of therapeutic communication, non-therapeutic communication, few ways to improved therapeutic communication, characteristics of therapeutic communication, barrier of THERAPEUTIC RELATIONSHIP, introduction of interpersonal relationship, types of IPR, elements/ dynamics of IPR, introduction of therapeutic nurse patient relationship, definition, purpose, elements/characteristics , and phases of therapeutic communication, definition of Johari window, uses, what actually model represent and its areas, THERAPEUTIC IMPASSES and its management in 5th semester Bsc. nursing and 2nd GNM studentsMeasuring, learning and applying multiplication facts.
Measuring, learning and applying multiplication facts.cgilmore6
╠²
║▌║▌▀Żs from a presentation by Professor Camilla Gilmore to the Association of Teachers of Mathematics and Mathematics Association Primary Interest group in June 2025.
This gave an overview of two studies that investigated children's multiplication fact knowledge. These studies were part of the SUM research project based at the University of Nottingham and Loughborough University. For more information see www.sumproject.org.ukABCs of Bookkeeping for Nonprofits TechSoup.pdf
ABCs of Bookkeeping for Nonprofits TechSoup.pdfTechSoup
╠²
Accounting can be hard enough if you havenŌĆÖt studied it in school. Nonprofit accounting is actually very different and more challenging still.
Need help? Join Nonprofit CPA and QuickBooks expert Gregg Bossen in this first-time webinar and learn the ABCs of keeping books for a nonprofit organization.
Key takeaways
* What accounting is and how it works
* How to read a financial statement
* What financial statements should be given to the board each month
* What three things nonprofits are required to track
What features to use in QuickBooks to track programs and grantsPlate Tectonic Boundaries and Continental Drift Theory
Plate Tectonic Boundaries and Continental Drift TheoryMarie
╠²
This 28 slide presentation covers the basics of plate tectonics and continental drift theory. It is an effective introduction into a full plate tectonics unit study, but does not cover faults, stress, seismic waves, or seafloor spreading.
To download PDF, visit The Homeschool Daily. We will be uploading more slideshows to follow this one. Blessings, Marie How to Manage Upselling of Subscriptions in Odoo 18
How to Manage Upselling of Subscriptions in Odoo 18Celine George
╠²
Subscriptions in Odoo 18 are designed to auto-renew indefinitely, ensuring continuous service for customers. However, businesses often need flexibility to adjust pricing or quantities based on evolving customer needs.How to Implement Least Package Removal Strategy in Odoo 18 Inventory
How to Implement Least Package Removal Strategy in Odoo 18 InventoryCeline George
╠²
In Odoo, the least package removal strategy is a feature designed to optimize inventory management by minimizing the number of packages open to fulfill the orders. This strategy is particularly useful for the business that deals with products packages in various quantities such as boxes, cartons or palettes. Battle of Bookworms 2025 - U25 Literature Quiz by Pragya
Battle of Bookworms 2025 - U25 Literature Quiz by Pragya Pragya - UEM Kolkata Quiz Club
╠²
Battle of Bookworms is a literature quiz organized by Pragya, UEM Kolkata, as part of their cultural fest Ecstasia. Curated by quizmasters Drisana Bhattacharyya, Argha Saha, and Aniket Adhikari, the quiz was a dynamic mix of classical literature, modern writing, mythology, regional texts, and experimental literary forms. It began with a 20-question prelim round where ŌĆśstar questionsŌĆÖ played a key tie-breaking role. The top 8 teams moved into advanced rounds, where they faced audio-visual challenges, pounce/bounce formats, immunity tokens, and theme-based risk-reward questions. From Orwell and Hemingway to Tagore and Sarala Das, the quiz traversed a global and Indian literary landscape. Unique rounds explored slipstream fiction, constrained writing, adaptations, and true crime literature. It included signature IDs, character identifications, and open-pounce selections. Questions were crafted to test contextual understanding, narrative knowledge, and authorial intent, making the quiz both intellectually rewarding and culturally rich. Battle of Bookworms proved literature quizzes can be insightful, creative, and deeply enjoyable for all.Overview of Off Boarding in Odoo 18 Employees
Overview of Off Boarding in Odoo 18 EmployeesCeline George
╠²
When an employee leaves the company, Odoo provides a streamlined offboarding process to ensure all necessary steps are taken. PEST OF WHEAT SORGHUM BAJRA and MINOR MILLETS.pptx
PEST OF WHEAT SORGHUM BAJRA and MINOR MILLETS.pptxArshad Shaikh
╠²
Wheat, sorghum, and bajra (pearl millet) are susceptible to various pests that can significantly impact crop yields. Common pests include aphids, stem borers, shoot flies, and armyworms. Aphids feed on plant sap, weakening the plants, while stem borers and shoot flies damage the stems and shoots, leading to dead hearts and reduced growth. Armyworms, on the other hand, are voracious feeders that can cause extensive defoliation and grain damage. Effective management strategies, including resistant varieties, cultural practices, and targeted pesticide applications, are essential to mitigate pest damage and ensure healthy crop production.LDMMIA GRAD Student Check-in Orientation Sampler
LDMMIA GRAD Student Check-in Orientation SamplerLDM & Mia eStudios
╠²
Completed Tuesday June 10th.
An Orientation Sampler of 8 pages.
It helps to understand the text behind anything. This improves our performance and confidence.
Your training will be mixed media. Includes Rehab Intro and Meditation vods, all sold separately.
Editing our Vods & New Shop.
Retail under $30 per item. Store Fees will apply. Digital Should be low cost.
I am still editing the package. I wont be done until probably July? However; Orientation and Lecture 1 (Videos) will be available soon. Media will vary between PDF and Instruction Videos.
Thank you for attending our free workshops. Those can be used with any Reiki Yoga training package. Traditional Reiki does host rules and ethics. Its silent and within the JP Culture/Area/Training/Word of Mouth. It allows remote healing but thereŌĆÖs limits for practitioners and masters. We are not allowed to share certain secrets/tools. Some content is designed only for ŌĆ£MastersŌĆØ. Some yoga are similar like the Kriya Yoga-Church (Vowed Lessons). We will review both Reiki and Yoga (Master symbols) later on. Sounds Simple but these things host Energy Power/Protection.
Imagine This package will be a supplement or upgrade for professional Reiki. You can create any style you need.
ŌÖźŌÖźŌÖź
ŌĆó* ╠ü ╠ł ╠¦.ŌĆó
(Job) Tech for students: In short, high speed is essential. (Space, External Drives, virtual clouds)
Fast devices and desktops are important. Please upgrade your technology and office as needed and timely. - MIA J. Tech Dept (Timeless)
ŌÖźŌÖźŌÖź
ŌĆó* ╠ü ╠ł ╠¦.ŌĆó
Copyright Disclaimer 2007-2025+: These lessons are not to be copied or revised without the
AuthorŌĆÖs permission. These Lessons are designed Rev. Moore to instruct and guide students on the path to holistic health and wellness.
ItŌĆÖs about expanding your Nature Talents, gifts, even Favorite Hobbies.
ŌÖźŌÖźŌÖź
ŌĆó* ╠ü ╠ł ╠¦.ŌĆó
First, Society is still stuck in the matrix. Many of the spiritual collective, say the matrix crashed. Its now collapsing. This means anything lower, darker realms, astral, and matrix are below 5D. 5D is thee trend. ItŌĆÖs our New Dimensional plane. However; this plane takes work ethic,
integration, and self discovery. ŌÖźŌÖźŌÖź
ŌĆó* ╠ü ╠ł ╠¦.ŌĆó
We donŌĆÖt need to slave, mule, or work double shifts to fuse Reiki lol. It should blend naturally within our lifestyles. Same with Yoga. ThereŌĆÖs no
need to use all the poses/asanas. For under a decade, my fav exercises are not asanas but Pilates. ItŌĆÖs all about Yoga-meditation when using Reiki. (Breaking old myths.)
Thank You for reading our Orientation Sampler. The Workshop is 14 pages on introduction. These are a joy and effortless to produce/make.Non-Communicable Diseases and National Health Programs ŌĆō Unit 10 | B.Sc Nursi...
Non-Communicable Diseases and National Health Programs ŌĆō Unit 10 | B.Sc Nursi...RAKESH SAJJAN
╠²
This PowerPoint presentation is prepared for Unit 10 ŌĆō Non-Communicable Diseases and National Health Programs, as per the 5th Semester B.Sc Nursing syllabus outlined by the Indian Nursing Council (INC) under the subject Community Health Nursing ŌĆō I.
This unit focuses on equipping students with knowledge of the causes, prevention, and control of non-communicable diseases (NCDs), which are a major public health challenge in India. The presentation emphasizes the nurseŌĆÖs role in early detection, screening, management, and referral services under national-level programs.
¤ö╣ Key Topics Included:
Definition, burden, and impact of NCDs in India
Epidemiology, risk factors, signs/symptoms, prevention, and management of:
Diabetes Mellitus
Hypertension
Cardiovascular Diseases
Stroke & Obesity
Thyroid Disorders
Blindness
Deafness
Injuries and Accidents (incl. road traffic injuries and trauma guidelines)
NCD-2 Cancers:
Breast Cancer
Cervical Cancer
Oral Cancer
Risk factors, screening, diagnosis, early signs, referral & palliative care
Role of nurse in screening, referral, counseling, and continuum of care
National Programs:
National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS)
National Program for Control of Blindness
National Program for Prevention and Control of Deafness
National Tobacco Control Program (NTCP)
Introduction to Universal Health Coverage and Ayushman Bharat
Use of standard treatment protocols and referral flowcharts
This presentation is ideal for:
Classroom lectures, field assignments, health education planning, and student projects
Preparing for university exams, class tests, and community field postingsjune 10 2025 ppt for madden on art science is over.pptx
june 10 2025 ppt for madden on art science is over.pptxroger malina
╠²
art science is over -talk by roger malina for jack madden groupExploring Ocean Floor Features for Middle School
Exploring Ocean Floor Features for Middle SchoolMarie
╠²
This 16 slide science reader is all about ocean floor features. It was made to use with middle school students.
You can download the PDF at thehomeschooldaily.com
Thanks! Marie 2025 June Year 9 Presentation: Subject selection.pptx
2025 June Year 9 Presentation: Subject selection.pptxmansk2
╠²
2025 June Year 9 Presentation: Subject selectionAd
MixturesandPureSubstances.ppt
- 1. DO:I will be able to explain the differences between pure substances and mixtures. EQ: 1. How do elements and compounds both qualify as pure substances? 2. Explain how to determine types of mixtures? 3. Compare and contrast pure substances and mixtures.
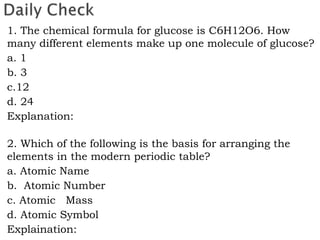
- 2. 1. The chemical formula for glucose is C6H12O6. How many different elements make up one molecule of glucose? a. 1 b. 3 c.12 d. 24 Explanation: 2. Which of the following is the basis for arranging the elements in the modern periodic table? a. Atomic Name b. Atomic Number c. Atomic Mass d. Atomic Symbol Explaination:
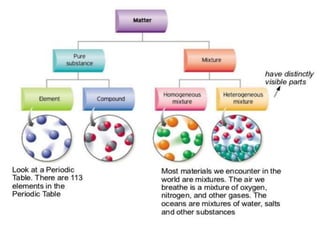
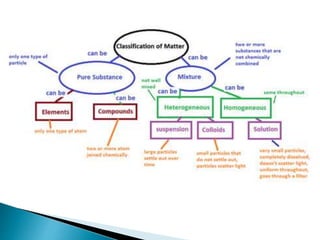
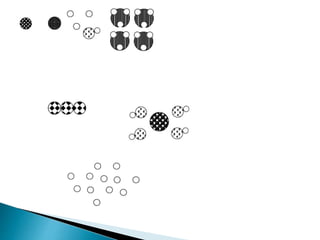
- 5. ELEMENTS COMPOUNDS Elements are the simplest pure substances. Examples: ŌĆó O-Oxygen ŌĆó H- Hydrogen ŌĆó Na- Sodium ŌĆó C- Carbon ŌĆó Fe- Iron ŌĆó Pb- Lead The smallest particle of an element that has the properties of that element is an atom. Compounds are pure substances that are made of more than one element bound together. Examples: ŌĆó H2O and CO2 A molecule is formed when two or more atoms chemically combine. HETEROGENOUS MIXTURES HOMOGENOUS MIXTURES All components of the mixture are visible because they do not mix together Particles not distributed evenly EX: sand and water vegetable soup oil and water Homogeneous mixtures Components cannot be distinguished from each other, appear as one substance Particles distributed evenly throughout EX: air, salt water, 10 karat gold *SOLUTIONS Pure Substance
- 6. Heterogeneous mixtures ’üĮ All components of the mixture are visible because they do not mix together ’üĮ Particles not distributed evenly EX: trail mix, vegetable soup, oil and water Homogeneous mixtures ’üĮ Components cannot be distinguished from each other, appear as one substance ’üĮ Particles distributed evenly throughout EX: air, salt water, 10 karat gold
- 8. ’üĮ Homogeneous mixtures are also called solutions. ’üĮ Separate particles are not visible because one dissolves in the other = dissolution ’üĮ In salt water, ŌŚ” salt is the solute, gets dissolved ŌŚ” water is the solvent, dissolves other substance
- 9. Q. Why do some substances dissolve and others do not? A. In a solute, each particle is attracted to each other to form a grain of it. When the solute is placed in a water, new attractive forces are present. If the attractive forces between the water and the solute are stronger than those holding the solute together, then the solute will break down and get dissolved in the water.
- 10. ’üĮ Because different amounts of solute can be dissolved in a solvent, we look at a solutionŌĆÖs SOLUBILITY. ’üĮ Definition: The maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature. ’üĮ Usually expressed as the number of grams of solute per 100mL of solvent.
- 11. ’üĮ Every chemical substance which dissolves in water has a fixed solubility. ŌŚ” If it does not dissolve, solubility = zero. ’üĮ Many of these solublities have been measured and special charts are produced displaying solubility of many substances at once.
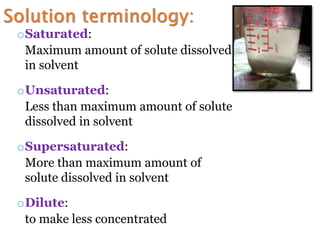
- 12. oSaturated: Maximum amount of solute dissolved in solvent oUnsaturated: Less than maximum amount of solute dissolved in solvent oSupersaturated: More than maximum amount of solute dissolved in solvent oDilute: to make less concentrated
- 13. 1. Sedimentation: occurs naturally when solid substances that are heavier than their solvent deposit at the bottom of the mixture. EX: Water treatment 2. Decantation: a heterogeneous mixture that has distinct layers can be separated by slowly pouring one of the layers into another container. EX: Separating cream from milk 3. Filtration: separates parts of a heterogeneous mixture by pouring it though a filter, the larger particles (residue) will be held in the filter while the smaller ones (filtrate) will pass through. EX: Brita 4. Distillation: used to separate components of a homogeneous mixture based on their different boiling points. Solution is heated and substance with lower boiling points evaporates and passes through a tube where it cools and turns back to water in another container.
- 15. DO:I will be able to explain the matter its molecular composition, characteristics, ability to change, and how combinations of elements and atoms from the different types of matter that make up the world. EQ: 1. How do elements and compounds both qualify as pure substances? 2. Explain how to determine types of mixtures? 3. Compare and contrast pure substances and mixtures.
- 17. ’üĮ An atom is to an element as a _____________ is to ____________. ’üĮ An atom is to a molecule as a _____________ is to ____________. ’üĮ An atom is to a compound molecule as a _____________ is to ____________.
- 18. DO:I will be able to explain the matter its molecular composition, characteristics, ability to change, and how combinations of elements and atoms from the different types of matter that make up the world. EQ: 1. How do elements and compounds both qualify as pure substances? 2. Explain how to determine types of mixtures? 3. Compare and contrast pure substances and mixtures.
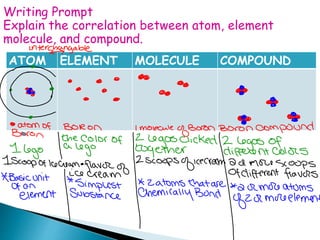
- 19. ATOM ELEMENT MOLECULE COMPOUND Writing Prompt Explain the correlation between atom, element molecule, and compound.
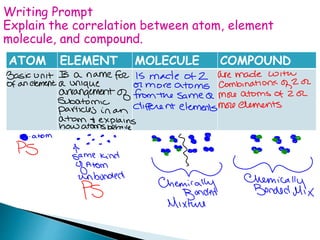
- 20. ATOM ELEMENT MOLECULE COMPOUND Writing Prompt Explain the correlation between atom, element molecule, and compound.