Monitoring of the additive manufacturing process for the use of biomaterials in medical field
- 2. 1. PURPOSES ? Monitoring the manufacturing process ? Evaluate disturbances ? Use biocompatible materials in medical products ? Evaluate the viability of the obtained product ? Generate reports for the 3D manufacturing process
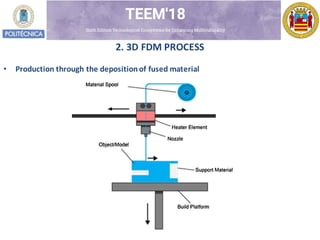
- 3. 2. 3D FDM PROCESS ? Production through the depositionof fused material
- 4. ? Geometrical Parameters of the material used (Diameter) ? Extrusion speed ? Humidity ? Temperature ? Vibrations 3. DISTURBANCES DURING THE PROCESS These disturbances affect the final quality of the product. In the case of biocompatible materials, they also affect their characteristics
- 6. 5. DISTURBANCES DURING THE PROCESS ? Extrusion speed: Volumes deposited in 10 mm of spool advance
- 7. 6. DISTURBANCES DURING THE PROCESS HUMIDITY ©C Density of the material can change (PLA is ) ©C The materials change their properties ? PLA-more breakable ? PVA can even melt
- 8. TEMPERATURE ? Extruder and chamber temperature ©C Correct adhesion ©C The materials change their properties Selected temperatur 7. DISTURBANCES DURING THE PROCESS
- 9. 8. ANALYSIS OF THE DISTURBANCES Humidity (%) Chamber temperature (?C) Extruder Temperature (?C) Filament Diameter (mm) Nominal Not controlled Not controlled 220 1,75 Average 31,64 25,56 218,28 1,78 Maximum 33,00 34,67 227,20 1,96 Minimum 31,00 19,04 213,53 1,61
- 11. 10. IMPORTANCE OF CONTROLLING DISTURBANCES ? In the case of manufacturing by 3D printing bio-ceramic joint implants, it has to be performed at low and controlled temperature ? The control of environmental conditions for 3d printing is of fundamental importance to controlthe quality of the product ? Specially in biomedical applications ? In many cases strict control of the temperature or humidity in the printing chamberis needed
- 12. 11. CONCLUSIONS ? Necessity to generate a report of each product to know the conditions in which it has been manufactured ? There are different disturbances in 3D FDM process that interfere in the quality and characteristics of the final product ? These disturbances could degrade bio materials ? It is necessary to control the parameters of the process to validate the final product