MP5040 Lecture 9.pdf- process Engineering
- 1. MP5040: PROCESS ENGINEERING Lecturer: Miss. J Sajeeva Lecture Number 09
- 2. BASIC PROCESS DEVICES ABSORPTION AND STRIPPING COLUMN
- 4. ABSORPTION VS STRIPPING 1. In absorption (also called gas absorption, gas scrubbing, or gas washing), there is a transfer of one or more species from the gas phase to a liquid solvent. 2. The species transferred to the liquid phase are referred to as solutes or absorbate. 3. Absorption involves no change in the chemical species present in the system. 4. Absorption is used to separate gas mixtures, remove impurities, or recover valuable chemicals.
- 5. ABSORPTION VS STRIPPING 1. The operation of removing the absorbed solute from the solvent is called stripping. 2. Absorbers are normally used with strippers to permit regeneration (or recovery) and recycling of the absorbent. 3. Since stripping is not perfect, absorbent recycled to the absorber contains species present in the vapor entering the absorber. 4. When water is used as the absorbent, it is normally separated from the solute by distillation rather than stripping
- 6. SUGGEST ANY DAILY ACTIVITIES THAT ARE USING ABSORPTION OR STRIPPING PROCESS
- 7. A TYPICAL ABSORPTION PROCESS 1. The feed, which contains air (21% O2, 78% N2, and 1% Ar), water vapor, and acetone vapor, is the gas leaving a dryer where solid cellulose acetate fibers, wet with water and acetone, are dried. 2. Acetone is removed by a 30-tray absorber using water as the absorbent. The percentage of acetone removed from the air stream is 99.5% (10.25/10.3). 3. Although the major component absorbed by water is acetone, there are also small amounts of oxygen and nitrogen absorbed by the water. 4. Water is also stripped since more water appears in the exit gas than in the feed gas. 5. The temperature of the exit liquid decreases by 3 â to supply the energy of vaporization needed to strip the water.
- 8. TYPES OF THE ABSORPTION AND STRIPPING COLUMNS 1. Absorption and stripping are conducted mainly in packed columns and plate columns (trayed tower).
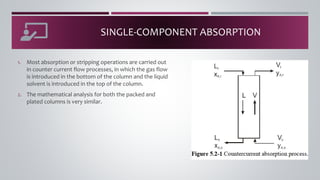
- 9. SINGLE-COMPONENT ABSORPTION 1. Most absorption or stripping operations are carried out in counter current flow processes, in which the gas flow is introduced in the bottom of the column and the liquid solvent is introduced in the top of the column. 2. The mathematical analysis for both the packed and plated columns is very similar.
- 10. MATERIAL BALANCE The overall material balance for a countercurrent absorption process ð¿ð + ðð¡ = ð¿ð¡ + ðð V = vapor flow rate L = liquid flow rate t, b = top and bottom of tower, respectively
- 11. COMPONENT MATERIAL BALANCE The component material balance for species A is ð¿ðð¥ð´ð + ðð¡ð¦ð´ð¡ = ð¿ð¡ð¥ð´ð¡ + ððð¦ð´ð ð¦ð´ = mole fraction of A in the vapor phase ð¥ð´ = mole fraction of A in the liquid phase
- 12. SOLUTE FREE COMPONENT MATERIAL BALANCE If the carrier gas is completely insoluble in the solvent and the solvent is completely nonvolatile, the carrier gas and solvent rates remain constant throughout the absorber. The material balance for solute A becomes à´¤ ð¿ à´¤ ðð´ð + à´¤ ð à´¤ ð ð´ð¡ = à´¤ ð¿ à´¤ ðð´ð¡ + à´¤ ð à´¤ ð ð´ð OR à´¤ ð ð´ð¡ = à´¤ ð¿ à´¤ ð à´¤ ðð´ð¡ + (à´¤ ð ð´ð â à´¤ ð¿ à´¤ ð à´¤ ðð´ð) à´¤ ðð´ = ð¥ð´ (1âð¥ð´) = ðððð ððððð¡ððð ðð ð´ ðð ð¡âð ðððð¢ðð ðððð ððððð¡ððð ðð ððð ð´ ððððððððð¡ð ðð ð¡âð ðððð¢ðð à´¤ ð ð´ = ð¦ð´ (1âð¦ð´) = ðððð ððððð¡ððð ðð ð´ ðð ð¡âð ð£ðððð ðððð ððððð¡ððð ðð ððð ð´ ððððððððð¡ð ðð ð¡âð ð£ðððð à´¤ ð¿ to denote the flow rate of the nonvolatile and à´¤ ð to denote the carrier gas flow rate,
- 13. SOLUTE FREE COMPONENT MATERIAL BALANCE The material balance for solute A can be applied to any part of the column. For example, the material balance for the top part of the column is à´¤ ð ð´ð¡ = à´¤ ð¿ à´¤ ð à´¤ ðð´ð¡ + (à´¤ ð ð´ â à´¤ ð¿ à´¤ ð à´¤ ðð´) In this equation, à´¤ ðð´ and à´¤ ð ð´ are the mole ratios of A in the liquid and vapor phase, respectively, at any location in the column including at the two terminals. This equation is called the operation line and is a straight line with slope à´¤ ð¿ à´¥ ð when plotted on à´¤ ðð´ and à´¤ ð ð´ coordinates.
- 14. HENRYâS LAW The equilibrium relation is frequently given in terms of the Henryâs law constant which can be expressed in many different ways: ðð´ = ð¾ð»ð¶ð´ = ðð¥ð´ = ðð¥ð´ In this equation, 1. ðð´ is the partial pressure of species A over the solution . 2. ð¶ð´ is the molar concentration with units of mole/volume. 3. The Henryâs law constant ð¾ð» and m have units of pressure/molar concentration and pressure/mole fraction, respectively. 4. K is the equilibrium constant or vapor-liquid equilibrium ratio.
- 15. HENRYâS LAW
- 16. EQUILIBRIUM CURVE â The relationship between solute concentration in the gas phase and in the liquid phase at constant temperature and pressure is known as the equilibrium distribution curve, as shown in the Figure below. â The presence of the solute in the liquid represent the its gas solubility at the prevailing temperature and pressure.
- 17. QUESTION A solute A is to be recovered from an inert carrier gas B by absorption into a solvent. The gas entering into the absorber flows at a rate of 500 kmol/h with ð¦ð´ = 0.3 and leaving the absorber with ð¦ð´ = 0.01. Solvent enters the absorber at the rate of 1500 kmol/h with ð¥ð´ =0.001. The equilibrium relationship is ð¦ð´ = 2.8 ð¥ð´ . The carrier gas may be considered insoluble in the solvent and the solvent may be considered nonvolatile. 1. Determine the à´¤ ð¿ and à´¤ ð 2. Find out the operation line 3. Construct the x-y plots for the equilibrium and operating lines using both mole fraction and solute-free coordinates à´¥ ðð¨ð = à´¤ ð³ à´¥ ð½ à´¥ ð¿ð¨ð + (à´¥ ðð¨ â à´¤ ð³ à´¥ ð½ à´¥ ð¿ð¨)
- 18. ANSWER Data ð¿ð¡ = 1500ðððð â , ð¦ð´ð = 0.3, ðð = 500ðððð â , ð¥ð´ð¡ = 0.001, ð¦ð´ð¡ = 0.01 ð¦ð´ = 2.8 ð¥ð´ (equilibrium curve) à´¤ ð¿ = ð¿ð¡ (1 - ð¥ð´ð¡) = 1500(1 â 0.001) = 1498.5 kmol/h à´¤ ð = ðð (1 - ð¦ð´ð) = 500(1 â 0.3) = 350 kmol/h
- 19. ANSWER The concentration of A in the solvent stream leaving the absorber can be determined from the following expressions: ð¥ð´ð = ð¹ððð¤ ððð¡ð ðð ð´ ðð ð¿ð ð¹ððð¤ ððð¡ð ðð ð´ ðð ð¿ð+à´¤ ð¿ ð¹ððð¤ ððð¡ð ðð ð´ ðð ð¿ð = Flow rate of A in ð¿ð¡ + Flow rate of A in ðð - Flow rate of A in ðð¡ ð¹ððð¤ ððð¡ð ðð ð´ ðð ð¿ð = ð¿ð¡ ð¥ð´ð¡ + ððð¦ð´ð- Flow rate of A in ð½ð
- 20. ANSWER But ð¦ð´ð¡ = ðððð ðððð ðð ð¨ ðð ð½ð ðððð ðððð ðð ð¨ ðð ð½ð+à´¥ ð = 0.01 ðððð ðððð ðð ð¨ ðð ð½ð = 3.5354 kmol/h
- 21. ANSWER Determine 1. à´¥ ðð¨ð 2. à´¥ ð¿ð¨ð 3. à´¥ ðð¨ð 4. à´¥ ð¿ð¨ð à´¤ ðð´ = ð¥ð´ (1âð¥ð´) = ðððð ððððð¡ððð ðð ð´ ðð ð¡âð ðððð¢ðð ðððð ððððð¡ððð ðð ððð ð´ ððððððððð¡ð ðð ð¡âð ðððð¢ðð à´¤ ð ð´ = ð¦ð´ (1âð¦ð´) = ðððð ððððð¡ððð ðð ð´ ðð ð¡âð ð£ðððð ðððð ððððð¡ððð ðð ððð ð´ ððððððððð¡ð ðð ð¡âð ð£ðððð