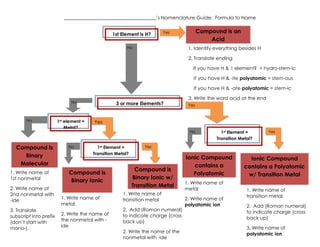
Naming flowchart
- 1. ______________________________________âs Nomenclature Guide: Formula to Name 1st Element is H? Compound is an Acid Yes No 1. Identify everything besides H 2. Translate ending If you have H & 1 element? = hydro-stem-ic If you have H & -ite polyatomic = stem-ous If you have H & -ate polyatomic = stem-ic No No Compound is Binary Molecular 1. Write name of 1st nonmetal 2. Write name of 2nd nonmetal with -ide 3. Translate subscript into prefix (donât start with mono-). 1st element = Metal? No 3 or more Elements? 3. Write the word acid at the end Yes Yes No 1st Element = Transition Metal? Compound is Binary Ionic 1. Write name of metal 2. Write the name of the nonmetal with ide 1st Element = Transition Metal? Yes Yes Compound is Binary Ionic w/ Transition Metal 1. Write name of transition metal 2. Add (Roman numeral) to indicate charge (cross back up) 2. Write the name of the nonmetal with -ide Ionic Compound contains a Polyatomic 1. Write name of metal 2. Write name of polyatomic ion Ionic Compound contains a Polyatomic w/ Transition Metal 1. Write name of transition metal 2. Add (Roman numeral) to indicate charge (cross back up) 3. Write name of polyatomic ion